
main content:
In terms of improving the cycle performance of spinel LiMn2O4, the use of impurity ions to stabilize the structure of spinel LiMn2O4 is considered to be one of the most effective methods to solve cycle capacity degradation. The choice of impurity ions directly affects the doping effect. There are many metal ions available for doping spinel LiMn2O4. Among the many doping ions, which ions may have better results?
1.Introduce cations with stable valence state
By introducing small and stable cations (such as Mg2+, A13+, Ga3+, etc.) to replace part of the Mn3+ in the spinel structure, to reduce the content of Mn3+ in the spinel, so as to achieve the purpose of inhibiting the Jahn-Teller effect.
The introduction of Al3+ makes LiAlyMn2-yO4 (y=0, 1/12, 1/9, 1/6, 1/3) in the y=1/12 sample cycle 200 times, the capacity retention rate is still 90%, lithium ion diffusion The coefficient has increased by an order of magnitude. However, A13+ doping causes a significant decrease in capacity. Doping Mg2+ can increase the discharge specific capacity, and the lithium ion diffusion coefficient can be increased to 10-8cm²/s, but as y in LiMgyMn2-yO4 (0≤y≤0.15) increases, the discharge specific capacity decreases. The above studies show that the introduction of cations such as Mg2+ and Al2+ into the spinel structure can increase the lithium ion diffusion coefficient.
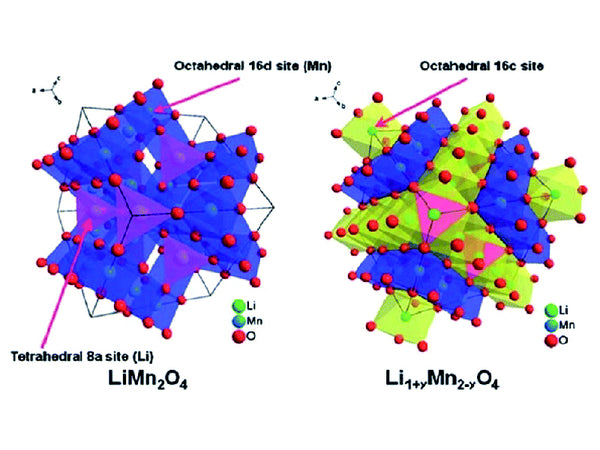
Spinel cation doping
2.Doping with variable valence cations
Doping a small amount of Ti, Fe, Co, Ni, Cu and other variable valence cations, although the spinel lithium manganese oxide 4V zone capacity is reduced, but its cycle stability is greatly improved. For example, doped with Fe cations, in Li0.9FexMn2-xO4 (0≤x≤0.2) and LiFexMn2-xO4 (0≤x≤0.5), Fe is dominated by high-spin Fe3+, and Fe4+ sequentially occupies the 16d position in the spinel structure , With the increase of y, the unit cell parameters of Fe doped products increase, and the thermal stability improves.
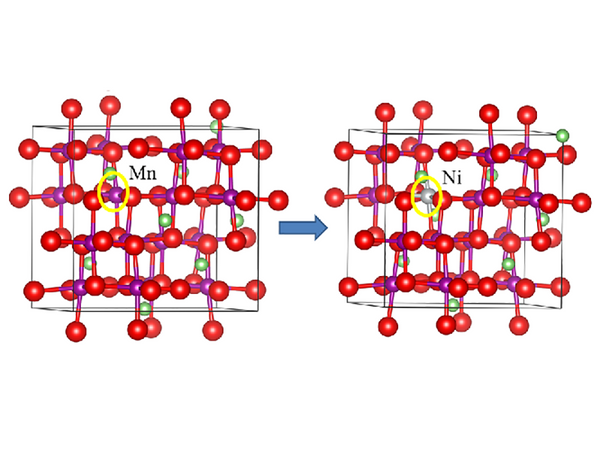
Ni-doped spinel LiMn2O4
Increasing the amount of nickel doping, LiNi0.5 Mn1.5O4 can obtain a discharge capacity of 114mA·h/g at 4.66V, and has a fairly good cycle stability. There is no Mn3+/Mn4+ redox couple in the 4V region in the cyclic voltammetry curve of the LiNi0.5 Mn1.5O4 thin film electrode. It exhibits a specific discharge capacity of 155mA·h/g at 4.7V, and the capacity retention rate is 50 cycles. 91%, increasing the amount of Cr, Co or Cu doping also appears similar, for example, the theoretical discharge capacity of LiCrMnO4 and LiCoMnO4 are 151mA·h/g and 145mA·h/g, respectively, and the specific discharge capacity is measured by experiment. It is 75mA·h/g and 95mA·h/g. In the CV diagram of LiCu0.5Mn1.5O4, a pair of obvious redox peaks also appeared in the high potential region 4.9V. The capacity of this peak is 1/3 of the total capacity. Since there is no decomposition of CuO, this The peak corresponds to the Cu2+/Cu3+ redox couple. Since the positive and negative materials of lithium-ion batteries are both lithium-intercalation compounds, if the negative electrode selects Li4Ti5O12 with a working voltage of 1.55V to form a Li4Ti5O12/LiM0.5Mn1.5O4 battery to obtain a stable 3V working voltage, it is possible to further improve the lithium-ion battery Based on this, this kind of 5V materials will have very good application prospects.
3.Anion doping
Anion doping can also stabilize the spinel structure. For oxygen ion doping, the ions are limited to monovalent or divalent anions whose properties are close to oxygen ions, such as F-, Cl-, S2-, etc. Since the atomic weight of F is smaller than that of oxygen, if an appropriate amount of F is added, the capacity of spinel LiMn2O4 can be increased. In addition, substituting monovalent ions for oxygen ion doping can reduce the average valence of manganese, which makes the range of cationic doping of manganese ions larger. Doping with Al and F can effectively improve the specific discharge capacity and cycle stability of LiAlyMn2-yO4-zFz (0≤y≤1/12, 0≤z≤0.04). The EIS results show that the electrochemical impedance of the material after doping with F is significantly reduced .

Fluorine-doped LiNi0.8Mn0.1Co0.1O2 for lithium-ion batteries
The type of doping ions, the amount of doping ions and the doping process are three important factors that determine the doping effect. The effects of anion and cation doping and composite doping are mainly reflected in the following aspects:
① When the valence state of the doped cation is lower than or equal to 3, the content of Mn3+ in the spinel will be reduced, the Jahn-Teller effect will be suppressed, and the stability of the spinel structure will be enhanced. The volume change of the spinel lithium manganese oxygen solid solution is reduced, thereby improving the cycle performance of the spinel LiMn2O4.
②By cation doping to make the lattice constant smaller, the distance between Mn3+ and Mn4+ will be shortened, thereby increasing DLi+ and electronic conductivity, and improving the high-current charging and discharging performance of LiMn2O4.
③ Both anion and cation doping will affect the redox potential of spinel LiMn2O4.
④Because transition metal ions have variable valence, when Ni, V, Cr, Cu and Co and other transition metal ions are doped, spinel LiMn2O4 will produce another discharge platform at about 5V.
⑤ The negative effect of cation doping is to reduce the capacity of the 4V region, but after doping, the structural stability of the solid solution is improved. It can be considered that the doped ions actually act as an indicator of overcharge and overdischarge. Before charging and over-discharging, the termination voltage is reached, thus ensuring the structural stability and cycle stability of the solid solution.