
There are many kinds of spinel lithium manganese oxides. In the research of lithium ion battery cathode materials, the main lithium manganese oxides that have received attention are spinel LiMn2O4, Li2Mn4O9 and Li4Mn5O12. Selecting different Li/Mn ratios and controlling the atmosphere and process conditions can obtain lithium manganese-oxygen solid solutions with different chemical compositions. Figure 1 is a phase diagram and a partial enlarged view of a manganese compound. It can be seen from Figure 1 that there are many compounds formed by lithium manganese oxide, and they can be transformed into each other under different conditions. This is the complexity of lithium manganese oxide as a cathode material. Figure 1(b) is an enlarged part of the MnO-Li2MnO3-λ-MnO2 phase region in Figure 1(a), and Figure (c) is an enlarged view of the shaded phase region in Figure (b). In the figure, the MnO-Li2MnO3 connecting line represents the stoichiometric rock salt component; the Mn3O4-Li4Mn5O12 connecting line represents the stoichiometric spinel component.
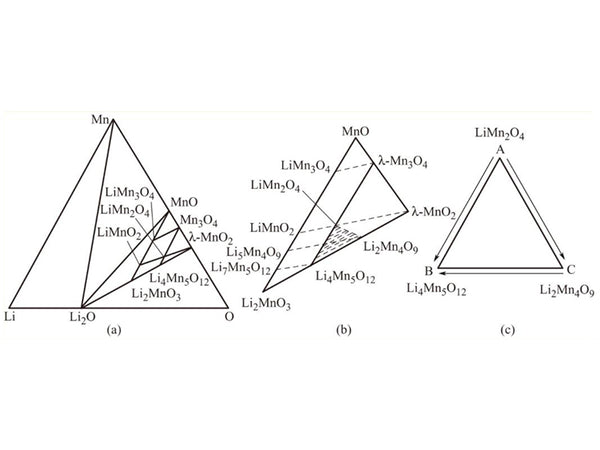
Figure 1 The oxide phase diagram of a lithium tank composed of spinel, defective spinel and rock salt structure
The triangular region composed of Mn3O4-Li4Mn5O12-λ-MnO2 is a defective spinel phase region, and lithium manganese oxide composed of any point in the region has a spinel structure.
The quadrilateral region formed by MnO-Li2MnO3-Li4Mn5O12-Mn3O4 is a defective rock salt (layered structure) phase region. The defective lithium manganese oxide component in the triangular area formed by LiMn2O4-Li4Mn5O12-MnO2 can be obtained by chemical delithiation of stoichiometric spinel lithium manganese oxide Li1+xMn2-xO4, so along the connecting line λ-MnO2-Li4Mn5O12 It means that Li2O and λ-MnO2 can form a completely mutually soluble solid solution. The LiMn2O4-λ-MnO2 connecting line represents the electrochemical delithiation process of LiMn2O4. The λ-MnO2-LiMn2O4-LiMnO2 connection line represents the electrochemical lithium insertion process and phase transition of LiMn2O4 .
Figure 1(c) shows the spinel structure of interest, which can be divided into metered spinel Li1+xMn2-xO4(0≤x≤0.33) and non-metered spinel. The latter includes two types: oxygen-rich (such as LiMn2O4+δ, 0<δ≤0.5) and hypoxia (such as LiMn2O4-δ, 0<δ≤0.14). In Figure 1(c), from A to C, the oxygen concentration increases, and from A to B, from C to B, the lithium ion concentration increases. It can be seen that the oxygen partial pressure and the lithium/manganese ratio have a direct impact on the composition and structure of the non-metered spinel. Oxygen-rich (such as LiMn2O4+δ, 0<δ≤0.5) and hypoxia (such as LiMn2O4-δ, 0<δ≤0.14) type spinel lithium manganese oxide have different values of δ, causing the average valence of manganese to change, thereby Make the non-metered spinel have different discharge specific capacity.
Strictly control the pressure and temperature of the atmosphere to make the reaction reach equilibrium, and stoichiometric compounds can be obtained. Therefore, to study non-stoichiometric compounds, such as the δ setting of LiMn2O4+δ, a series of stoichiometric compounds can be obtained by controlling a certain oxygen partial pressure and temperature. With the help of TG, XRD and elemental analysis are used to determine the non-stoichiometric δ value in the non-stoichiometric compound structure model. Spinel lithium manganese oxide with different lithium content will show different chemical reaction activity and electrochemical performance due to different chemical positions.
The spinel-type compound LiMn2O4 cathode material, in which the anion lattice contains cubic densely packed oxygen ions, is similar in structure to α-NaFeO2, except for the distribution of cations at the octahedral position and the tetrahedral position. The discharge process is mainly divided into two steps, that is, around 4V, and the other platform is below 3V, as shown in Figure 2.
Charging: LiMn2O4→Mn2O4+Li (embedded in negative electrode, such as graphite carbon)
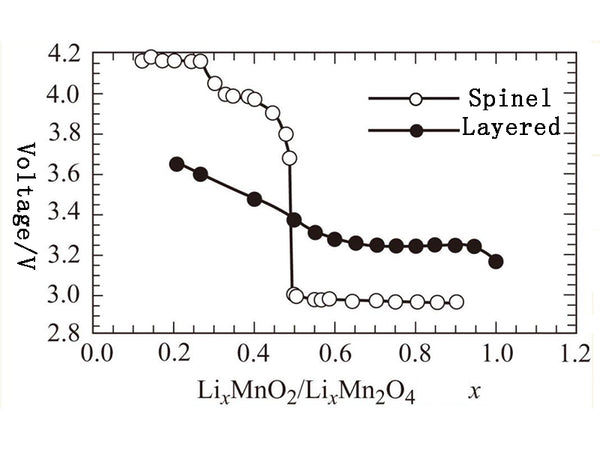
Figure 2 Voltage characteristics of lithium-manganese composite oxide materials with different doping levels
The intrinsic defect of spinel lithium manganese oxide mainly refers to the situation where Li and O deviate from the stoichiometric ratio. Because the synthesis conditions have a great influence on the content of Li, Mn, and O in LiMn2O4, it is very important to control various processes in the synthesis process. The first discharge capacity of stoichiometric spinel is high, up to 140mA·h/g, but in the high voltage area of x<0.45 during the cycle, the coexistence of unstable two-phase structure causes the capacity attenuation; non-stoichiometric spinel Although the initial capacity of Li1+yMn2O4+δ is low, only 110~120mA·h/g, the cycle stability is very good, and the desorption/intercalation of lithium is a homogeneous reaction. The stoichiometric spinel exhibits capacity loss during the cycle, which causes Mn to enter the 8a position, which turns the metered spinel into a defective spinel, and vacancies appear at the 16d position. Correspondingly, in the high voltage region of x<0.45 , The charge and discharge curve is changed from L type to S type. This shows that both lithium excess and oxygen enrichment are beneficial to improve the structure and stability of the solid solution.
Based on the results of DSC and in-situ XRD, some people analyzed the reason why Li1+yMn2-yO4-δ changed from a cubic structure to a tetragonal structure during cooling to 210K, and believed that the existence of oxygen defects is the only necessary condition for phase transition. The synthesis temperature, heat treatment time, cooling method, etc. can all cause different degrees of oxygen defects, with different oxygen defect concentrations and different phase transition temperatures. The metered spinel LiMn2O4 has no oxygen defects, so there is no phase transition. With the increase of oxygen defects, the average valence of Mn decreases, the unit cell parameter a increases, and the phase transition temperature increases. Low-temperature baking of LiMn2O4 in an argon atmosphere may produce two types of point defects: oxygen defects or interstitial manganese ions. If the oxygen-deficient samples are fired again in an oxygen atmosphere, the original structure can be restored. This is the low-temperature baking of LiMn2O4 in an argon atmosphere. , Produced a direct proof of oxygen deficiency. It can be seen that processes such as synthesis temperature, heat treatment time, and cooling methods can all cause oxygen defects to varying degrees.
The cycle performance of the material is determined by its cubic lattice parameter value, and the cubic lattice parameter value is related to the average valence state of manganese. For example, the cubic lattice parameter value is about 0.823nm, this value is consistent with the lattice parameter value of the lithium-rich material Li1+xMn2-xO4; in the lithium-rich material, the average valence of manganese is about 3.58; the valence is a numerical value The material can reduce the dissolution of manganese and prevent the Jahn-Teller distortion effect caused by Mn3+. The related values of the lattice parameter a0 in the chemical composition Li1+xMn2-xO2 are shown in Figure 3(a). The general formula for calculating the parameters is a0=8.4560-0.21746x.
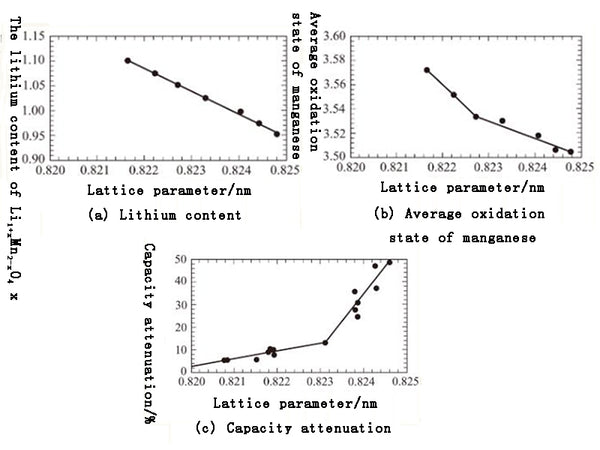
Figure 3 Influence of different conditions on the lattice parameters of spinel Li1+xMn2-xO4
The lattice parameters can be used to indirectly calculate the oxidation state of manganese, as shown in Figure 3(b). In addition, Figure 3(c) clearly shows the influence of the lattice parameters on the capacity loss rate after the first 120 cycles. At the same time doping with aluminum ions and fluoride ions can maintain the stability of the cycle capacity at high temperatures, such as Li1+xMn1-x-yAlyO4-zFz. Here, the value of y and 2 is 0.1~0.3. In addition, if the voltage on the spinel surface is kept higher than the formation voltage of the Li2Mn2O4 phase, the reaction of Mn3+ to Mn2+ on the uneven surface will decrease, and the balance of the following equation will shift 2Mn3+↔Mn2++Mn4+ to the left. Since divalent manganese ions are easily dissolved in the acid electrolyte, it is necessary to prevent the formation of divalent manganese ions. Once it is dissolved in the electrolyte, the divalent manganese ions will diffuse to the cathode and be reduced to manganese metal at the cathode , Thereby depleting lithium ions and reducing the electrochemical capacity of the battery.
Spinel-type compounds are the research and development hotspot of high-energy lithium-ion battery cathode materials used in hybrid electric vehicles (HEV), although their capacity can only reach about 90mA·h/g at high rates. This type of material will self-discharge when it is fully charged, especially at high temperatures. LiBOB can be used to replace the LiPF6 electrolyte, which is easily decomposed to generate HF in a wet state, to solve this technical problem. In the spinel used, lithium replaces part of the manganese to obtain a stable structure, such as Li1.06Mn1.95Al0.05O4. An alternative method is to coat the surface of spinel particles with certain materials, such as ZrO2 or AlPO4, which can also serve to adsorb HF.
Spinel Li4Ti5O12 can also be used as a cathode material for high-energy batteries. Its charging voltage (lithium insertion) is about 1.55V. Therefore, there is no danger when lithium metal deposition occurs on graphite carbon at high rates. When the magnification is as high as 12C, the nanostructure and microstructure of the material coexist at 60℃. If the electrode is combined with a high-rate cathode material such as composite layered oxide or spinel LiMn2O4, it can be prepared with low cost and safety A good working voltage is a 2.5V battery; it can also be combined with olivine LiFePO4, the resulting battery has excellent cycling performance at 1.8V, and a battery with no capacity loss after 100 cycles; if combined with high-potential spinel, the battery voltage It can reach 3.5~4V.
In terms of improving the cycle performance of spinel LiMn2O4, the use of impurity ions to stabilize the structure of spinel LiMn2O4 is considered to be one of the most effective methods to solve cycle capacity degradation. The choice of impurity ions directly affects the doping effect, and there are many metal ions available for doping spinel LiMn2O4.