What is the lithium insertion behavior of graphite materials?
In graphite crystals, the carbon atoms in the layers are superimposed on the metal bonds by covalent bonds and are firmly bonded to each other, and the layers are connected only by weak van der Waals forces. This special structure makes graphite have special chemical properties. Some atoms, molecules or ions can be inserted between the layers of the graphite crystal without destroying the two-dimensional network structure, but only increasing the layer spacing to generate graphite-specific compounds. Known as Graphite intercalation compound (GIC), GIC can be divided into two categories: electrostatic attraction type and covalent bond type, according to the different binding relationship between the insert (guest) and the hexagonal network plane layer (host).
The emergence of lithium-ion batteries is based on the principle that the graphite host can be intercalated by guest lithium atoms. After the intercalation of lithium atoms, the sp2 hybrid orbital of carbon atoms in the graphite layer remains unchanged, the plane remains flat, there are free electrons, and lithium atoms It can donate electrons, which also increases the electrons in the graphite layer, so it has higher conductivity. At the same time, the graphite layer and the embedded layer are arranged in parallel, and are regularly inserted every other layer, two layers, and three layers. They are called first-order, second-order, third-order…graphite interlaminar compounds, respectively.
When graphite is used as the negative electrode of a lithium-ion battery, lithium intercalation reaction occurs to form a compound LixC6 of different levels. For intact crystalline graphite, a first-order compound is finally formed with the intercalation of lithium, and its structure is shown in Figure 1.
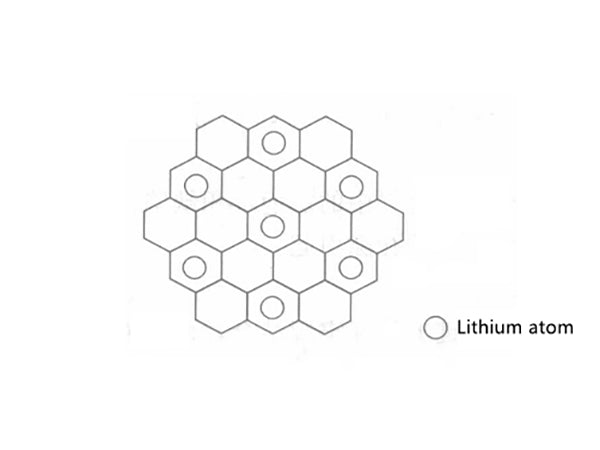
Figure 1 Plane structure of graphite intercalation compound
Lithium occupies the position next to the six-membered ring in LiC6, so it can be calculated that its theoretical capacity is 372mA·h/g. After lithium is inserted into graphite, the interlayer spacing also changes accordingly, from 0.3354nm when no lithium is inserted to 0.3706nm. When other carbon materials are used as the negative electrode, lithium intercalation reaction will also occur, but the intercalation mechanism may be different from that of graphite. .
Regarding the lithium intercalation process of graphite, a representative model proposed by Ohzuku et al., in this model, believes that there are four levels of lithium intercalation compounds in the process of lithium intercalation in graphite, and there are two plane lithium densities to form six types Different phases, namely LiC6 type LiC6, LiC12 phase, LiC9 type LiC9, LiC18 and higher order phases.
The structures of these compounds of the same order may be different. In order to distinguish the compounds of the same order with different ratios, we call LiC9 type quasi-order compounds. The lithium-graphite system undergoes a step-to-step change according to the following steps:
Area (I) LiC12 (second order) LiC6 (first order)
Area (Ⅱ) LiC18 (quasi second order) LiC12 (second order)
Area (Ⅲ) LiC36 (quasi fourth order) LiC27 (quasi third order) LiC18 (quasi second order)
Area (IN) Graphite LiC36 (quasi-fourth order)
The lithium intercalation mechanism of graphite can be determined by electrochemical reduction. There are two basic methods: constant current method and cyclic voltammetry. In comparison, cyclic voltammetry, especially slow scan cyclic voltammetry (SSCV), is more effective. Figure 2 shows the changes in the potential and composition of graphite during the lithium insertion process measured by the constant current method. The appearance of the potential platform indicates the existence of the two-phase region.
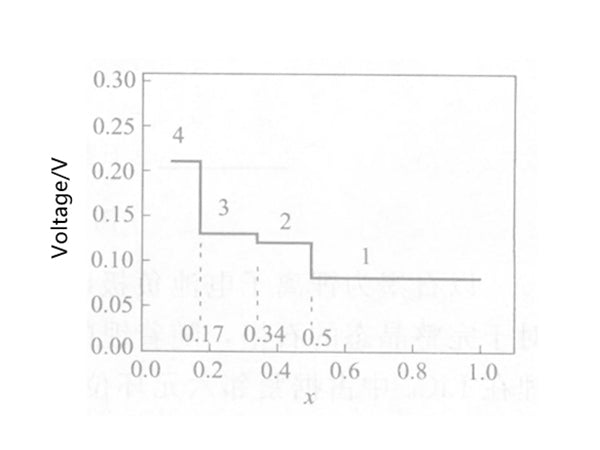
Figure 2 Changes in potential and composition of graphite during lithium insertion
The diffusion rate of lithium in graphite is also an important indicator of the performance of graphite lithium insertion, because it directly determines the charge and discharge rate of lithium-ion batteries. The diffusion rate of lithium in graphite is 10-10~10-11 cm2/s, which is 2 orders of magnitude smaller than that in coke, and the conductivity is similar to that of graphite. The lithium intercalation kinetics of graphite is also obvious. The anisotropy, the speed of lithium ions through the boundary surface is about 80 times the speed through the base surface. Therefore, the insertion of lithium in graphite is generally performed from the end surface, but if the base surface has structural defects like micropores, the insertion behavior can also be performed from the base surface.
The lithium insertion characteristics of graphite-like carbon materials are: low and flat lithium insertion potential, which can provide high and stable working voltage for lithium-ion batteries; high lithium insertion capacity, with a theoretical capacity of 372mA·h/g; but it is compatible with organic solvents Poor tolerance, solvent co-intercalation is easy to occur.