main content:
- (1) Li2 mechanism of lithium molecule
- (2) Multilayer lithium mechanism
- (3) Lattice lattice mechanism
- (4) Elastic ball elastic net model
- (5) Layer-side-end-surface lithium storage mechanism
- (6) Lithium storage mechanism of nanometer graphite
- (7) Carbon-lithium-oxygen mechanism
- (8) Molecular mechanism of single-layer graphite flakes
- (9) Mechanism of microporous lithium storage
The lithium storage mechanism of lithium in graphite, that is, lithium is inserted into graphite to form a graphite intercalation compound; however, there are many methods for the lithium storage mechanism of amorphous carbon materials, mainly lithium molecular Li2 mechanism, multilayer lithium mechanism, and lattice lattice mechanism , Elastic ball-elastic net model, layer edge surface mechanism, nano-scale graphite lithium storage mechanism, carbon-lithium-hydrogen mechanism, single-layer ink sheet molecular mechanism and microporous lithium storage mechanism.
(1) Li2 mechanism of lithium molecule
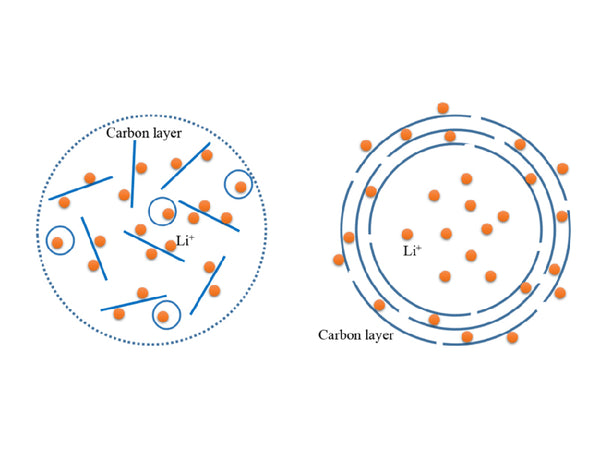
In lithium-intercalated PPP-700 (a carbon material in which poly-p-phenylene is pyrolyzed at 700°C), lithium exists in two forms, one is an ionic state, exists as an interlayer compound, and is located in the hexagonal aromatization In the center of the carbon ring, six carbon atoms correspond to one lithium ion; another type of lithium atom exists as a molecule Li2, and the atoms are connected by covalent bonds (Figure 1), that is, lithium is not only inserted into the next carbon ring in an ionic state to form Graphite intercalation compounds can also enter the nearest carbon ring in the form of Li2 molecules. Due to the existence of Li2 molecules, its reversible capacity reaches 1116mA·h/g, which is up to the level of LiC2, which is three times that of graphite materials, and its volumetric specific capacity is larger than that of metallic lithium.
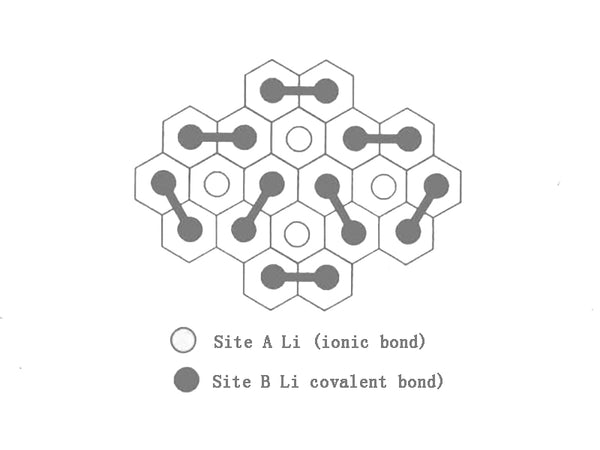
Figure 1 Schematic diagram of two forms of lithium in pyrolytic carbon materials
(2) Multilayer lithium mechanism
The mechanism of multilayer lithium believes that the high reversible lithium storage capacity (410mA·h/g) of mesophase carbon microspheres is attributed to the different positions of lithium, and its main contribution is the formation of multilayer lithium. Its structure is shown in Figure 2. The first layer of lithium occupies the α position as shown in the figure; in fact, this layer of lithium is a graphite intercalation compound, which is thermodynamically and kinetically stable. In order to make the distance between Li atoms lower than covalent lithium (0.268nm), another layer of lithium had to be formed at the 3 position. Of course, the effect between the β layer and the graphite layer is significantly lower than that of the α layer. At the same time, in order to reduce the electrostatic repulsion between the α and β layers, there is a certain covalent effect between them. In the same way, a third layer of lithium is formed at the γ position. At a lower potential, it helps the formation of multilayer lithium, but at the same time can lead to the formation of dendrites, thereby reducing cycle life.
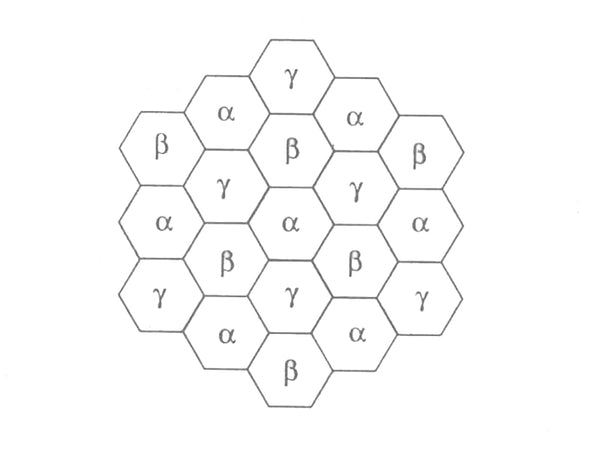
Figure 2 Structure model of multilayer lithium deposited on the α and β planes of carbon materials
(3) Lattice lattice mechanism
Phenolic resin is cracked to obtain a polyacetylene semiconductor material PAS. It is amorphous, but it has obvious 002 diffraction peak, its interlayer spacing d002 is 0.37~0.40nm, the reversible lithium insertion capacity can reach 530mA·h/g, and its maximum reversible capacity It can reach 1000mA·h/g, which is equivalent to the level of LiC2 compound. In the lattice structure of lithium metal, there are 46 lithium atoms in a 1nm cubic lattice. The atomic and ionic radius of lithium is 0.153nm and 0.06nm, respectively, and its reversible lithium storage exceeds 372mA·h/g, indicating that lithium can be inserted in a more compact way in carbon materials than in graphite materials. The research results show that after the carbon material with d002 of 0.40nm stores lithium, neither the atomic state nor the ionic state of lithium exist. It can be seen from Figure 3 that d002 is a 0.40nm PAS carbon material, and 47 lithium atoms can be stored in a 1nm cubic lattice.
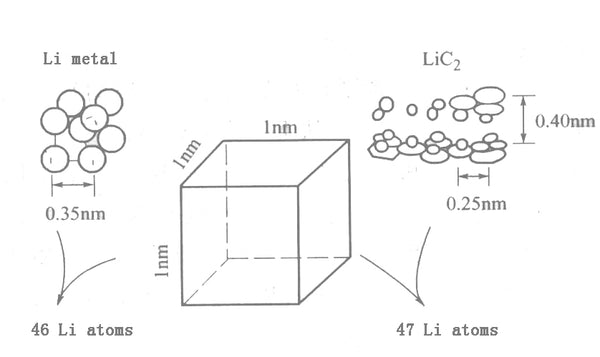
Figure 3 Schematic model of metallic lithium and PAS doped to LiC2 level
(4) Elastic ball elastic net model
The elastic ball-elastic net model is mainly based on the one-dimensional compression theory and certain assumptions. It determines the existence of the corresponding insert by calculating the pressure required for the existence of the insert compound, Thus, the amount of lithium inserted into the amorphous carbon material can be obtained.These basic assumptions include: the insert (metal) is an elastic sphere, its initial radius is the radius of the metal atom, and the compression coefficient is the same as that of the metal; the graphite plane is an elastic net, which can squeeze the insert. The coefficient of elasticity of the mesh is equivalent to that of the graphite a-axis; each graphite intercalation compound system has its characteristic parameter σig. It corresponds to the effective pressure to shrink the insert and expand the graphite mesh.
Based on the above basic assumptions and the one-dimensional compression theory k1P=-Δl/l0, the expression of the minimum pressure P required for the stable existence of the intercalation compound is as follows:
P=(8r3m-a3min)/(8kMr3m-σig)
In the formula, P——the minimum pressure required for the stable existence of the insert CnM;
rm——metal atomic radius;
amin——The shortest distance between M layers in CnM;
kM——The compressibility of metal;
σig——Characteristic parameter of the seam.
The above expression can be used to infer the minimum pressure of some intercalating compounds, such as C2M, C6M, and C8M, which is basically consistent with the experimental results, especially the C2Li and C2Na systems.
(5) Layer-side-end-surface lithium storage mechanism
The amorphous carbon material made from ribbon like carbon film (RCF) has a reversible lithium storage capacity of 440mA·h/g. The crystal structure of carbon materials has a relatively large impact on its reversible lithium storage capacity. Different crystal structures lead to different mechanisms of action of lithium and carbon materials. Therefore, it can be considered that there are three main methods for reversible lithium storage of carbon materials: ①Carbon materials Although the crystal parameters La and Lc of carbon materials are relatively small, there is still a part of the graphite structure, so lithium can be inserted into the interlayer to form a traditional graphite intercalation compound; ②The edge reaction of the carbon material, because the carbon material is without Shaped, so there are many defects. And lithium can react with the carbon atoms at the ends. This kind of interaction is similar to the effect of polyethylene doped with lithium, which can reach the level of C3Li; ③The surface reaction of carbon materials, lithium can react with the carbon atoms on the surface, this kind of reaction is similar to the above-mentioned side-end reaction . However, this reaction does not lead to an increase in the distance between graphite layers. The reversible storage of lithium in the latter two methods is called doped lithium, while the reversible storage of lithium in the former method is called inserted lithium.
(6) Lithium storage mechanism of nanometer graphite
PAS carbon material is obtained by heat treatment of phenolic resin, and its reversible capacity can reach 438mA·h/g. The Raman spectrum of carbon material mainly has two sets of peaks, one is located near 1350cm-1, and the other is located near 1580cm-1. The former is due to the formation of nano-scale graphite crystals, that is, the resulting graphite particles are very small, only a few nanometers; while the latter is caused by the formation of graphite crystals, which are much larger than the former, so they are called D2 peak and G2 peak. From the change of the ratio of the intensity of the D2 peak and the G2 peak with temperature, there is a large peak near 700°C, which is consistent with the capacity change of the obtained carbon material. It can be considered that there are several different phases in the obtained carbon material: graphite phase, nanometer graphite phase and other phases. Before the pyrolysis temperature of 700°C, the nano-graphite phase is mainly formed, and after 700°C, the graphite phase is mainly formed, that is, the nano-graphite phase and other phases transform to the graphite phase. Although nano-scale graphite has a small size, it can not only store lithium reversibly like graphite, but also store lithium on the surface and edges, so its lithium storage capacity is larger than graphite. In the carbon material obtained at 700°C, the content of nano-scale graphite is the largest, so its reversible lithium storage capacity is the largest at 700°C.
(7) Carbon-lithium-oxygen mechanism
Cracking a variety of materials near 700℃, such as petroleum coke, polyvinyl chloride, polyvinylidene fluoride, etc. The reversible lithium storage capacity of the obtained carbon material is related to the H/C ratio, which increases with the increase of the H/C ratio. The same is true even if the H/C ratio is as high as 0.2. It is believed that lithium can bond with hydrogen atoms in these hydrogen-containing carbon materials. This kind of bonding is the covalent transfer of part of the electrons on the 2s orbital from the inserted lithium to the adjacent hydrogen atom, and at the same time the C-H bond is partially changed. This bonding is an activation process, which results in a significant lag in the potential when lithium is released. When lithium is released, the original C-H bond is restored. Failure to fully recover will result in a continuous decrease in cycle capacity.
Some people think that lithium may react with the C-H bond as follows:
C-H+2Li=C-Li+LiH
C-H+2Li=C-Li+(1/2)H2
(8) Molecular mechanism of single-layer graphite flakes
The structure of hard carbon materials prepared at about 1000°C is very different from the graphite layered structure. In their structure, single layers of carbon atoms are disorderly and closely connected to each other. Like a large number of scattered cards, this material has a very low In this material, lithium can be adsorbed on both sides of each graphite layer, resulting in the insertion of more lithium. As shown in Figure 4.
Figure 4 Molecular mechanism of single-layer graphite flakes
The lithium molecule Li2 mechanism and the multilayer lithium mechanism, as a result, each six-membered ring can store a lithium atom; the lattice lattice mechanism is to explain the structure of LiC2 from crystallography; in addition, the layer edge -Surface reversible lithium storage mechanism and nano-scale graphite reversible lithium storage mechanism are mutually inclusive to a certain extent. However, all the above-mentioned explanations have their obvious shortcomings. For example, the mechanism of lithium molecule Li2 is amorphous compared to carbon materials, so the structure of carbon materials cannot have a regular planar structure like graphite; at the same time, the preparation of LiC2 must be carried out under high pressure (greater than 1.52109Pa).
(9) Mechanism of microporous lithium storage
The mechanism of microporous lithium storage was first proposed by A. Mabuchi et al. After treating MCMB at different temperatures, it was found that the discharge capacity of MCMB obtained by heat treatment at 700°C was as high as 750mA·h/g. Pores have a lot to do. The proposed microporous lithium storage mechanism is shown in Figure 5. The schematic diagram shows that lithium is simultaneously doped into the micropores during the process of inserting into the carbon layer, and in the process of lithium extraction, first from the carbon layer Lithium is removed and then removed from the micropores through the carbon layer.
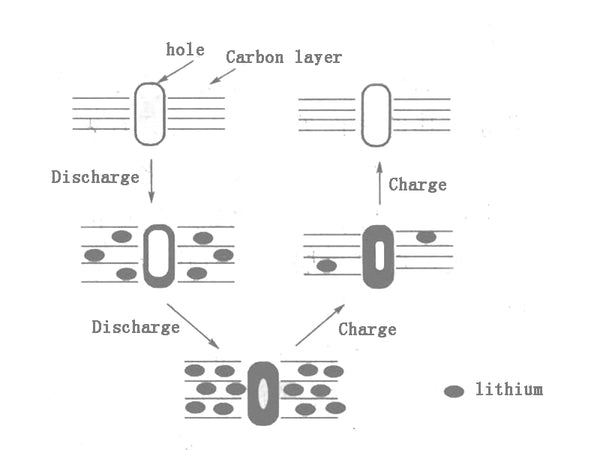
Figure 5 Schematic diagram of charging and discharging of microporous lithium storage mechanism
Compared with the improved model, the biggest difference lies in the location or source of the micropores. The new micropore mechanism believes that most of the micropores are located in the carbon layer, not between the carbon layers. The micropores are mainly formed by defects caused by the escape of small molecules during the carbonization process. Therefore, these micropores are unstable and change with the reversible insertion and extraction of lithium, which leads to a continuous decrease in the reversible lithium storage capacity as the number of cycles increases.
The process of lithium insertion and extraction in amorphous carbon materials is as follows: firstly, lithium is inserted into the graphite crystallites, and then inserted into the micropores located in the middle of the graphite crystallites to form lithium clusters or lithium molecules Lix (x≥2); When lithium is extracted, lithium is first extracted from the peripheral graphite crystallites, and then the lithium clusters or lithium molecules located in the micropores are extracted through the graphite crystallites. This can reasonably explain the voltage close to 0V during lithium insertion and the voltage hysteresis during deintercalation: after the insertion of lithium in the micropores in the graphite microcrystals, the voltage is around 0V, and during deintercalation, there are defects around the micropores The structure, there are free radical carbon atoms, and the force is relatively strong with lithium. Therefore, a certain force is required for lithium to escape from the micropores, so a voltage hysteresis phenomenon occurs.
The change of interlayer spacing d002 in the process of lithium insertion and extraction is also consistent with this mechanism: when lithium is inserted, d002 increases and reaches 0.37nm, and then does not change with the insertion of lithium; while during extraction, d002 first 0.37nm decreases and does not change with the release of lithium when it reaches a certain value.
The explanation of the capacity decay due to the mechanism of microporous lithium storage is: during the cycle, due to the unstable defect structure around the micropores, the lithium insertion and extraction process lead to the destruction of these structures. Due to the destruction of the carbon structure, the reversible capacity is attenuated.