
|
Main content: |
Fuel cell electric vehicles use fuel cells as power sources, which are energy-saving, pollution-free, and environmentally friendly vehicles. It is one of the important technical routes for future vehicle development. The reaction process of the fuel cell includes: hydrogen gas reaches the anode through pipes or gas guide plates; under the action of the anode catalyst, one hydrogen molecule is decomposed into two hydrogen atoms and two electrons are released; at the other end of the cell, oxygen (or Air) reaches the cathode through pipes or air guide plates, while hydrogen ions pass through the electrolyte to the cathode, and electrons also reach the cathode through the external circuit; under the action of the cathode catalyst, oxygen and hydrogen ions react with electrons to form water, and the electrons are in the external circuit. The main components of the fuel cell are as follows.

1) Electrode
The electrode of the fuel cell is the place where the electrochemical reaction of the fuel oxidation reaction and the oxidant reduction reaction occurs. The key to its performance lies in the performance of the catalyst, the material of the electrode and the manufacturing process of the electrode. Electrodes can be divided into anodes
(anode) and cathode (cathode) two parts, the thickness is generally 200~500mm.
2) Electrolyte membrane
The main function of the electrolyte separator is to separate oxidants and reductants, and conduct ions, so the thinner the electrolyte separator, the better. As far as the current technology is concerned, its thickness is generally about tens of μm to hundreds of μm; There are two development directions. One is to first make porous diaphragms with insulating materials such as asbestos (asbestos) membranes, silicon carbide (SiC) membranes, and lithium aluminate (LiAlO3) membranes, and then immerse them in molten lithium potassium carbonate and potassium hydroxide. With phosphoric acid, it is attached to the pores of the diaphragm; the other is the use of perfluorosulfonic acid resin (such as PEMFC) and yttria-stabilized zirconia YSZ (such as SOFC).
3) Current collector
The current collector is also known as a bipolar plate (bipolar has functions such as collecting current and diverting reactive gases. The performance of the current collector mainly depends on its material properties and its processing technology.
1.Classification of fuel cells
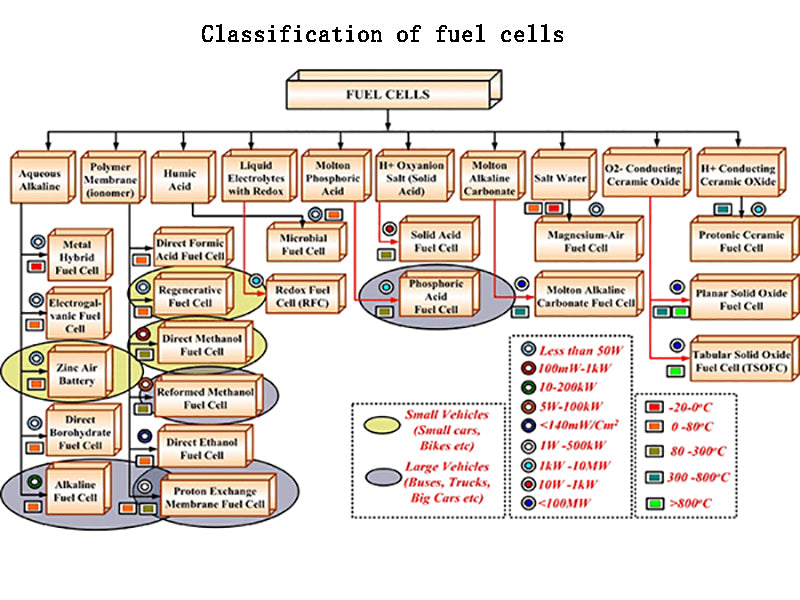
Fuel cells can be classified in the following ways.
(1) Classified by working temperature.
Fuel cells can be divided into low temperature fuel cells and high temperature fuel cells. Low temperature fuel cells include alkaline fuel cells (AFC, operating temperature at 100 ℃), solid polymer type proton membrane fuel cell PEMFC, also known as proton membrane fuel cell, operating temperature is within 100 ℃) and phosphoric acid fuel cell (PAFC) The working temperature is 200℃); high temperature fuel cells include molten carbonate fuel cells (MCFC, working temperature is 650℃) and solid oxide fuel cells (SOFC, working temperature is 1000℃).
(2) Classify according to the order of appearance of technology.
PAFCs can be referred to as first-generation fuel cells, MCFCs as second-generation fuel cells, and SOFCs and PEMFCs as third-generation fuel cells.
(3) According to the different classification of electrolyte types.
Fuel cells can be divided into alkaline type, phosphoric acid type, polymer type, molten carbonate type, solid electrolyte type, etc.
2.Several types of fuel cells that are currently being studied more
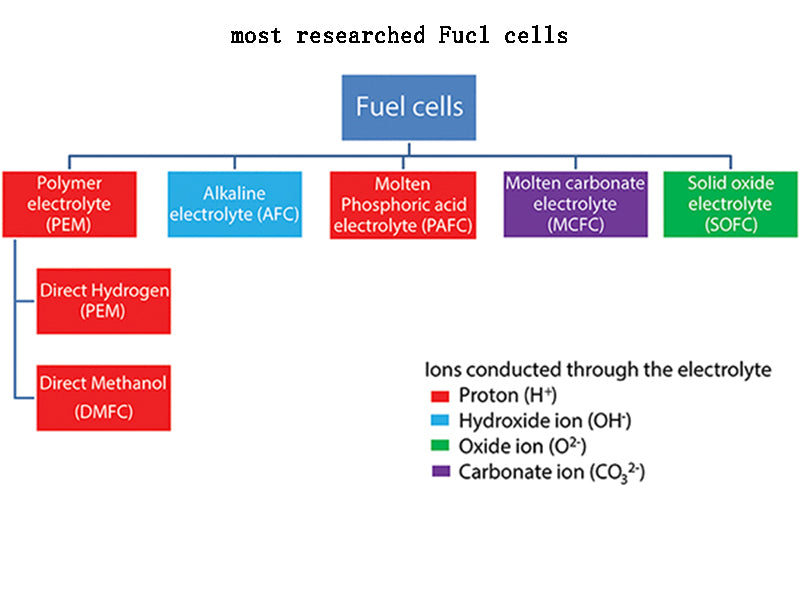
(1) Alkaline fuel cell (AFC).
The battery uses alkaline solution as electrolyte, such as aqueous potassium hydroxide (KOH) solvent as electrolyte. In an alkaline fuel cell, the conductive ions are OH- fuel and hydrogen, and the reaction formula is:
Anode reaction: 2H2+4OH-→4H2O+4e- (standard electrode potential is -0.828V)
Cathodic reaction: O2+2H2O+4e-→4OH- (standard electrode potential is 0.401V)
Reaction: O2+2H2→2H2O
Theoretical electromotive force is 0.401V+0.828V=1.229V
Alkaline fuel cells can achieve higher efficiency, especially the oxidation reaction of oxygen (O2→OH-) is easier than the reduction reaction of oxygen in acid fuel cells, so its activity loss is lower. Since the air contains carbon dioxide (CO2), if the electrolyte is in contact with the gas containing CO2, carbonate ions will be generated in the electrolyte, which will affect the output power of the fuel cell. Therefore, a CO2 removal device must be equipped, but this will lead to high costs. increase.
(2) Proton exchange membrane fuel cell (PEMFC).
The monomer of the battery consists of an anode, a cathode and a proton exchange membrane. The anode is where the hydrogen fuel is oxidized, the cathode is where the oxidant is reduced, and the proton exchange membrane is used as the electrolyte. Both electrodes contain catalysts that accelerate the electrochemical reaction of the electrodes. For DC power supply, the anode is the negative pole of the power supply, and the cathode is the positive pole of the power supply. The reactions that take place in PEMFC are:
Fuel electrode: H2→2H++2e-
Air pole: 1/2O2+2e-+2H+→H2O
Overall reaction: H2+(1/2)O2→H2O
In the fuel electrode, the supplied fuel gas H2 is decomposed into H+ and e-, H+ moves to the electrolyte and reacts with the oxygen supplied from the air electrode side, and e- reaches the air electrode side through an external load circuit. In order to obtain a certain output power, it is necessary to use the method of stacking multiple layers of components to obtain a higher voltage. The electrolyte in the PEMFC is a membrane of a proton-conductive polymer-based material, and a porous carbon body is generally used as the electrode. It is worth noting that the CO component in the fuel gas can cause catalyst poisoning and reduce the electrode performance. For this reason, the CO content in the fuel gas must be limited in the application of PEMFC.
(3) Phosphoric acid fuel cell (PAFC).
The battery uses liquid phosphoric acid as the electrolyte, which is usually located in a silicon carbide matrix. The operating temperature of phosphoric acid fuel cells is slightly higher than that of proton exchange membrane fuel cells and alkaline fuel cells, at 150°C-200°C, but still requires a platinum (Pt) catalyst to accelerate the reaction. The reactions at the anode and cathode are the same as in the PEM fuel cell, but because of its higher operating temperature, the reaction rate at the cathode is faster than that in the PEM fuel cell. The higher operating temperature of PAFC also makes it more tolerant to impurities. Phosphoric acid fuel cells are less efficient than other fuel cells, and their heating time is longer than that of proton exchange membrane fuel cells. In addition to using hydrogen as fuel, PAFC can also directly use low-cost fuels such as methanol, natural gas, and city gas to participate in the reaction. Compared with the Alkaline Hydrogen Oxygen Fuel Cell, its biggest advantage is that it does not require CO2 treatment equipment.
(4) Molten carbonate fuel cell (MCFC).
The cell is a high-temperature fuel cell (500°C to 800°C), which relies on molten carbonate (usually lithium potassium carbonate or lithium sodium carbonate) to conduct ions. The ion being conducted is the carbonate ion (CO32-). The electrode reaction of molten carbonate fuel cell is different from other types of fuel cells, and its reaction formula is:
Anode: H2+CO32-→H2O+CO2+2e-
Cathode: 1/2O2+CO2+2e-→CO32-
The main advantage of high temperature fuel cells is that they can directly process hydrocarbon fuels, while the high temperature operating environment also increases kinetic rates and reduces the reliance on catalysts. However, because carbonate is an alkaline substance and is extremely corrosive at high temperatures, fuel cells will consume a certain amount of fuel during the heating process, and the high temperature operating conditions make it difficult to use them in electric vehicles. .
(5) Solid oxide fuel cell (SOFC).
The cell conducts ions (1000°C~1200°C) in a ceramic separator. Generally, the ceramic material is responsible for conducting oxygen ions (O2-) for YSZ, and its conduction mechanism is similar to that of semiconductors, which are often called solid-state components, and its name is derived from this similarity. The semi-reaction formula is as follows:
Anode: H2+O2-→H2O+2e-
Cathode: 1/2O2+2e2-→O2-
The biggest advantage of solid oxide fuel cells is that their electrolytes are always in the solid state during operation. Compared with molten carbonate fuel cells, the disadvantages of solid oxide fuel cells and the environment in which they operate at high temperatures may affect their safety of use and fuel economy.
Fuel cell technology is a hot spot in the competition of major international auto companies. GM in the United States, Toyota, Honda, Nissan in Japan, and Daimler in Germany all have their own fuel cell technology for vehicles. These technologies also represent the current international fuel cell technology. advanced level. The existing technology can make the stack power reach 1500-2000W/L, and the dynamic life can reach 4000-5000h. At present, the focus of research and development in the fuel cell industry is mainly to reduce costs and improve the power density, working life, reliability and environmental adaptability of fuel cell systems.
In addition to power density and lifetime, cost is also one of the main bottlenecks restricting the commercialization of fuel cell vehicles. The commercial price of a fuel cell system is as high as $3,000 to $5,000 per kilowatt. If it is calculated based on a system with an annual output of 500,000 units (sets) of 50kW, the target price of the system in 2003 announced by the US Department of Energy is $225/kW. The price difference is large. Even at the 2008 target price of $73/kW in the US Department of Energy's report, fuel cells still cost 2.5 times the cost of an internal combustion engine vehicle. Among them, the fuel cell stack accounts for 47% of the total cost of the fuel cell system, and other parts such as air supply, hydrogen supply, water management, cooling, thermal management, assembly testing, etc. account for 53%. The U.S. Department of Energy requires fuel cell costs to be controlled to a level comparable to that of internal combustion engine vehicles, or $30/kW, by 2017. In addition, the energy conversion efficiency of fuel cell vehicles should continue to improve. Toyota Motor Corporation found that if the overall efficiency of "from mine to wheel" is considered, the total efficiency of hybrid vehicles is 28%, and the current total efficiency of fuel cell electric vehicles. 29%, while the report proposes that the total energy conversion efficiency of fuel cell electric vehicles is expected to increase to 42% in the future. At the same time, we must also realize that the development of fuel cell vehicles is a huge systematic project. It is not only a revolution in automobile technology, but also involves planning and construction of large-scale hydrogen production, hydrogen storage and refueling stations and other related infrastructure. How to reasonably coordinate the development of infrastructure and fuel cell electric vehicles is also very important.