Supercapacitors (electric double layer capacitors) are a new type of energy storage element that utilizes the interface electric double layer formed between the electrode material and the electrolyte to store energy. When the electrode material is in contact with the electrolyte, due to the interaction of intermolecular force, Coulomb force or interatomic force between the interfaces, an interfacial electric double layer will appear at the solid-liquid interface, which is a stable double-layer charge with opposite sign. For an electrode-solution system, the system will form an electric double layer at the solid-liquid interface due to the electronic conductivity of the electrode and the ionic conductivity of the electrolyte solution. When an external electric field is applied to the two electrodes, the anions and cations in the solution will migrate to the positive and negative electrodes respectively under the action of the electric field, and an electric double layer will be formed on the surface of the electrodes; when the applied electric field is removed, the positive and negative charges on the electrodes and the ions with opposite charges in the solution will attract each other and make the electric double layer more stable, which will generate a stable potential difference between the positive and negative electrodes. For a certain electrode in the system, an anisotropic ionic charge equivalent to the charge on the electrode will be generated within a certain distance on the electrode surface to keep it electrically neutral; when the two electrodes are connected to an external power source, a corresponding current is generated in the external circuit due to the transfer of charges on the electrodes, and the migration of ions in the solution into the solution will show electrical neutrality, which is the charge/discharge principle of supercapacitors. Compared with traditional capacitors and secondary batteries, the specific power of supercapacitors is more than 10 times that of batteries, and the ability to store electric charges is higher than that of ordinary capacitors. The energy storage process of supercapacitors is reversible because it is achieved by electrochemically polarizing the electrolyte solution, and the whole process does not produce electrochemical reactions. The working principle of supercapacitor is shown in Figure 1.
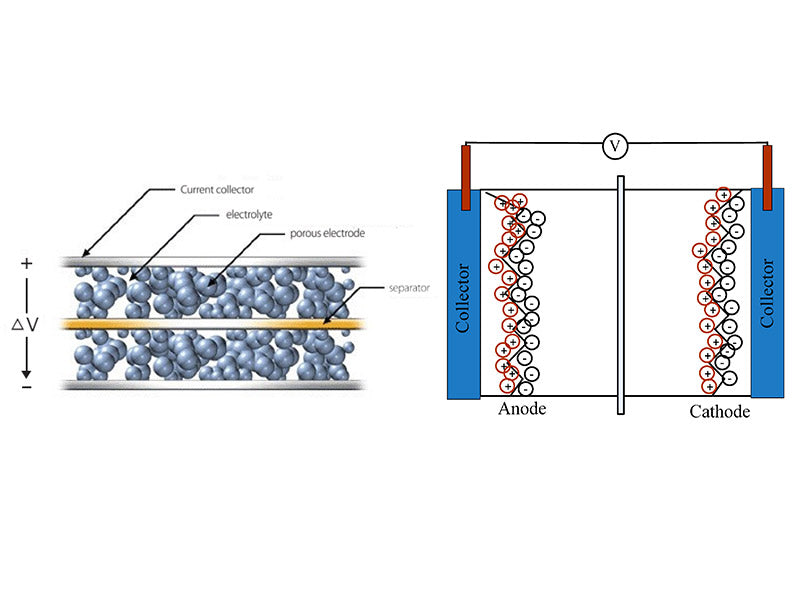
Figure 1 - The working principle of supercapacitors
In theoretical research, the helmholtz model is usually used to approximate the supercapacitor. The monolayer of electrons on the electrode side and the monolayer of ions on the solution side form an electric double layer, which is similar in structure to a plate capacitor. The density of excess charges on the electrode side is equal to the density of excess charges on the solution side. The charge density q is proportional to the interface potential difference V generated by the electric double layer and inversely proportional to the thickness d of the electric double layer, that is,
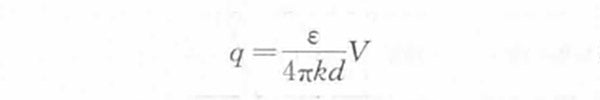
Then the electric double layer differential capacitance per unit area
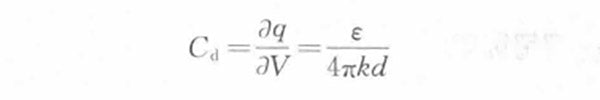
(1-2)
The electrostatic capacity of the electric double layer capacitor can also be obtained from the formula (1-1)
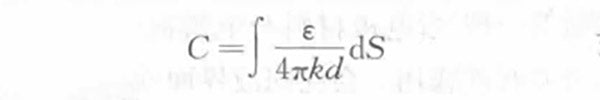
(1-3)
In the formula,
S —— the actual surface area of the electrode that can form an electric double layer in the system, mm2;
ε—— the dielectric constant of the electrolyte solution;
d ——The thickness of the electric double layer, which is the distance from the ion center to the electrode surface, and its size determines the size and concentration of the ions in the electrolyte. When the concentration of the electrolyte solution is high, d is usually 0.5~1.0nm.
It can be seen from formula (1-1) that the electrostatic capacitance C of the supercapacitor is inversely proportional to the thickness d of the electric double layer, and proportional to the actual area S formed by the electrodes. In order to make the electric double layer capacitor store more charges, according to the above corresponding relationship, the available surface area of the polarized electrode should be as large as possible, and the ions present in the electrolyte should be as close as possible to the area of the polarized electrode. In addition, since each unit electric double layer capacitor is composed of two electrodes, it can be regarded as a series combination of two capacitors. Therefore, the relationship between the amount of electricity Q stored in the electric double layer capacitor, the voltage U and the electrostatic capacity C is
Q=1/2CU (1-4)
and the energy stored in the electric double layer capacitor is
W=QU=1/2CU2 (1-5)
The electrode material is the main factor that determines the capacity of the capacitor. The electrode material is required to have high electrical conductivity and no chemical reaction with the electrolyte. The surface area is as large as possible, the price is cheap, and it is easy to shape during the preparation process. At present, the representative of supercapacitor electrode materials is RuO2·nH2O, and the specific capacitance has reached 720F/g, but Ru resources are scarce and expensive. The low-cost, high-surface-area porous carbon electrode material has a specific capacitance value of only about 200 F/g. Supercapacitors have been developed for more than half a century. As early as 1957, Becker in the United States has applied for a patent on the use of activated carbon as an electrode material for supercapacitors. Nippon Electric Co., Ltd. (NBC) produced the first supercapacitor named Supercapacitor and successfully brought it to the market with the storage backup power supply as the application object. Since then, the United States, Japan, Germany, South Korea and China have all carried out the production of supercapacitors and rapidly promoted the development of supercapacitors. The key materials of supercapacitors mainly include electrode materials, electrolytes, separators and current collectors, etc., while the research on supercapacitors mainly focuses on electrode active materials.

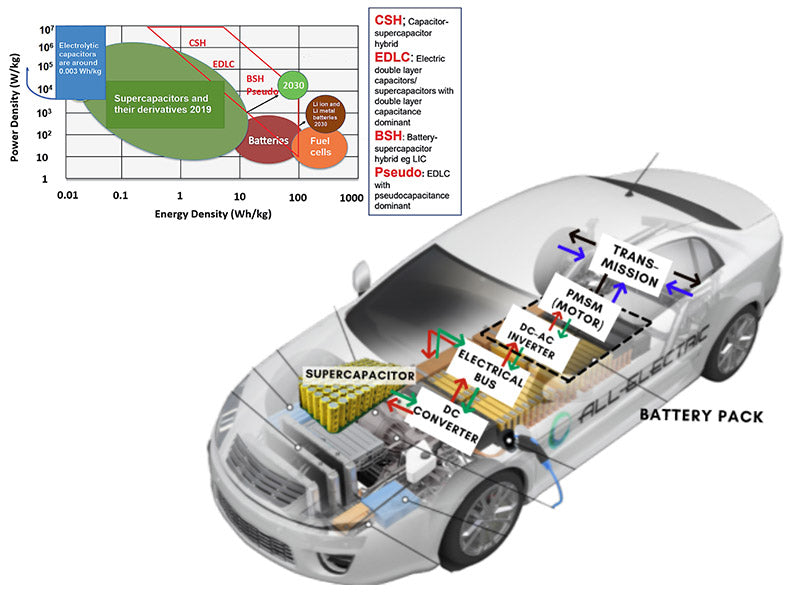
According to the requirements of the US Department of Energy, the specific energy and specific power of supercapacitors used in EVs and HEVs exceed 15Wh/kg and 1600W/kg, while the specific energy of current typical supercapacitors is about 2Wh/kg. Since supercapacitors can perform high-power charging/discharging, they can be applied to other vehicles, such as the use of supercapacitors to store the braking energy of urban rail vehicles or large passenger cars to provide peak power output during acceleration. In addition, judging from the current battery applications, the mixed use of lithium-ion power batteries and supercapacitors has certain prospects. Because of the high energy density of lithium-ion batteries and the high power density of supercapacitors, especially in urban rail vehicles or large passenger cars, their braking moment will generate greater energy feedback than small electric vehicles. And when the vehicle starts off or accelerates, its tractive power is greater than that required by a small electric car. High-power charging and discharging can be achieved through supercapacitors, and when continuous energy consumption is required under constant speed conditions, lithium-ion power batteries can be used to complete energy conversion. At present, supercapacitors are mainly used in hybrid electric vehicles, and the supercapacitors developed by Maxwell in the United States have been well used in many types of electric vehicles. The composite power system composed of super capacitor and battery is considered to be one of the effective ways to solve the power problems of electric vehicles in the future.