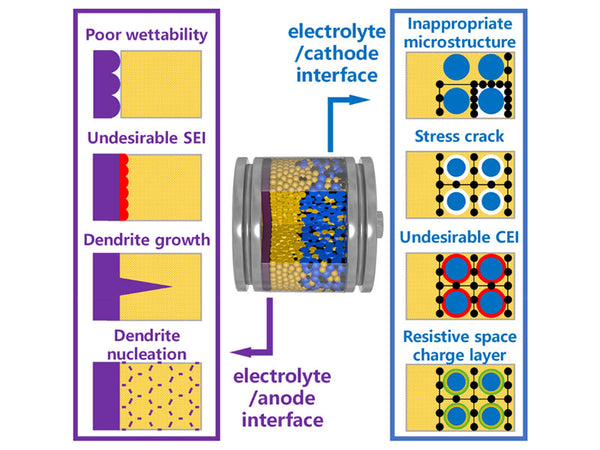
Many commercial lithium-ion batteries use flammable and volatile solvents, which may leak and cause fires. This is especially true for large-capacity, high-voltage, and high-energy-density lithium-ion batteries. In order to solve this problem, one of the effective methods to produce a more safe and reliable lithium-ion battery is to replace the flammable organic liquid electrolyte with a non-flammable solid electrolyte. The development of solid electrolytes with high ion conductivity and reduction of electrode/electrolyte interface impedance are the prerequisites for improving all-solid-state lithium-ion batteries. Due to the characteristics of single cation conductivity, fast ion transport, and high thermal stability, inorganic solid electrolytes are the most promising electrolyte materials for all-solid-state lithium-ion batteries.
The basic requirements for solid electrolytes are: the ionic conductivity is high (room temperature), the electronic conductivity is negligible, the structure is stable in a larger temperature range, and the contact with the positive and negative electrodes is stable and reliable in a larger charge and discharge voltage range.
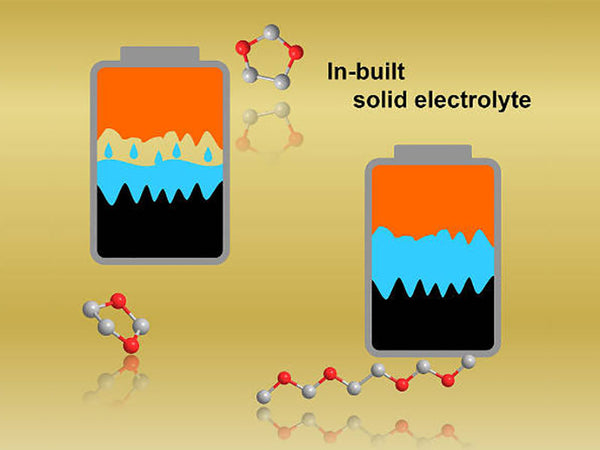
For most solid materials, such as most ionic crystal materials, they only have high ionic conductivity when they are in a liquid state (solution or high-temperature melting). In a solid state, they are almost completely unable to conduct ions. Solid electrolyte refers to a material that has relatively high ionic conductivity (similar to that of molten salt or liquid electrolyte) when in a solid state, and is called fast ionic conductor and super ionic conductor. This concept is often used to refer to some solid substances whose ionic conductivity is similar to that of liquid electrolytes or molten salts.
The history of solid electrolytes can be traced back to the emergence of stable aluminum oxide luminophores (called Nerst luminophores in 1897) discovered by Nerst at the end of the 19th century that can conduct oxygen ions. However, in the following half a century, people did not understand the conduction mechanism of ionic crystals until Wagner elaborated on it in one of his papers in 1943. Due to the development of solid physical chemistry, many new solid-state ion conduction phenomena have been discovered and studied. The main findings include various alkali metal halide ion conductors, among which the epoch-making solid electrolyte is silver iodide (AgI) discovered by Tubandt et al. That is, when solid silver iodide changes from β phase to α phase at 149°C, its ionic conductivity suddenly becomes almost as high as that of liquid AgI. Thus, α-AgI became the first "super ionic conductor". Since then, due to the extraordinary ionic conductivity of α-AgI, extensive research has been carried out on it from the aspects of physics and crystal chemistry. In 1935, Strock pointed out from the crystallographic point of view that the Ag in α-AgI (two Ags in each cell) is statistically distributed in 42 equivalent gaps in a body-centered cubic composed of anions (I-). This shows that the cation (Ag+) lattice is a molten state. Although some details of this explanation are still controversial, it is believed that the high Ag+ conductivity of α-AgI comes from a specific crystal structure such as α-AgI. At the same time, Joffe proposed the concept of lattice defects or interstitial ions from the perspective of crystallographic defect theory. According to the thermodynamic theory of ion transport, in the mid-1960s, people designed and synthesized a solid electrolyte material RbAg4I5 with an ion conductivity close to that of an electrolyte solution. Another successful example is the famous sodium ion conductor NASICON (Na1+xZr2P3-xSixO12) designed and synthesized by Goodenough et al. in 1967. This is a solid electrolyte that is completely tailored to the structure of the material based on the conduction mechanism of ions in the three-dimensional tunnel structure as understood by people in crystal chemistry. This successful material design soon led to the birth of many new solid ion conductors. However, the most important discovery since the 1950s may be the β-Al2O3 with high sodium ion conductivity synthesized by Kunmer et al. (the ideal composition is Na2O·11Al2O3).
Since the 1980s, due to the needs of energy conversion and storage, many new solid electrolyte materials have been synthesized and extensively and deeply studied, including oxygen ion conductors, fluoride ion conductors, silver ion conductors and copper ion conductors, sodium ion conductors and potassium ion conductors, proton conductors and lithium ion conductors. At present, solid electrochemical devices with the preparation of solid electrolyte materials as the core are forming a new class of high-tech, including high-energy density batteries, ceramic membrane fuel cells, solid electrochemical sensors, high-temperature membrane reactors, and electrochemical catalysis. Solid-state ionology developed based on the fast ion conductivity of inorganic solids has become an important branch of modern materials science and solid-state chemistry.