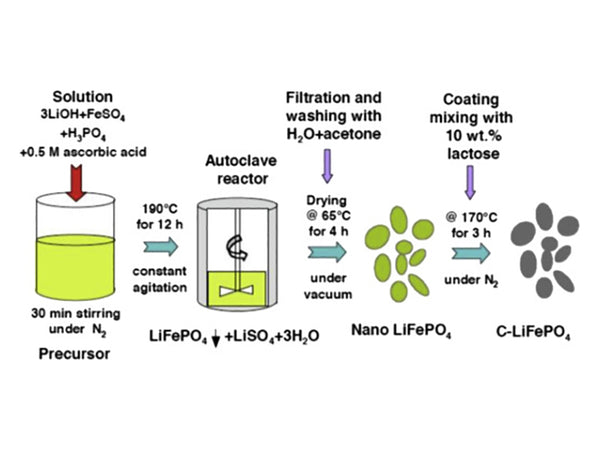
Among the examples of hydrothermal synthesis of lithium-ion battery materials, the typical one is the synthesis of LiFePO4.
Generally, it is to mix lithium source compound, iron source compound, phosphorus source compound, doping element compound or conductive agent, etc., react for 0.5-24h in a closed stirred reactor at 5~120℃, after filtering, washing and drying The nano-precursor is obtained, and then the precursor is placed in a high-temperature furnace, in a non-air or non-oxidizing atmosphere, and calcined at a constant temperature of 500-800° C. for 5-18 hours to prepare lithium iron phosphate nano powder.
The formation mechanism of LiFePO4 can also be studied by hydrothermal method. Using FeSO4·7H2O, H3PO4 and LiOH (the ratio of substances is 1:1:3) as raw materials, LiFePO4 product can be obtained from the reaction system at 33.5MPa. When the reaction temperature is lower than 120℃, the product obtained is Fe(PO4)·8H20 and a mixture of LiFePO4. When the temperature rises to 300℃, all the phosphates disappear, and the product obtained is pure olivine-type LiFePO4.That is to say, in the process of preparing LiFePO4 by hydrothermal method, it must go through the intermediate product Fe(PO4)·8H20 stage. When the reaction temperature rises to 400°C, the resulting product has a finer particle size and a more regular morphology. Studies have found that the higher the reaction temperature, the larger the specific surface area of the product. The reaction time has no obvious effect on the morphology of the product, but the sample reacted for 10 minutes has a smaller particle size and more uniform distribution than the sample reacted for 1 to 5 hours. When the reaction temperature is not higher than 200℃, the reaction temperature and time have almost no effect on the pH value of the liquid after the reaction, both are about 9.22. When the pH value reaches a certain level, Li3PO4 and Fe2O3 will appear in the product. , Fe3O4 and other miscellaneous phases.
Figure 1 shows the comparison of XRD patterns of LiFePO4 prepared by hydrothermal method, solid phase method and co-precipitation method.
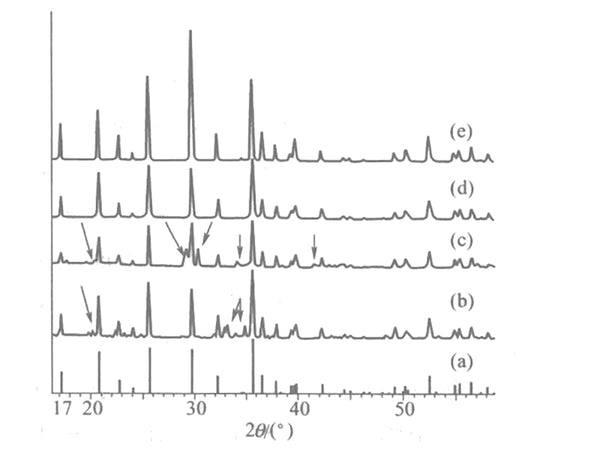
Figure 1 XRD patterns of LPePO4 prepared by different methods
(a) Pure LiFePO4 crystal; (b) Solid phase method;
(c) Co-precipitation method; (d) Hydrothermal method; (e) Preparation by mechanochemical method (the miscellaneous peak in the figure is marked with an arrow)
The figure (a) is the XRD pattern of pure phase LiFePO4 crystal. (b) is LiFePO4 prepared by the solid-phase method. It can be seen that there is a certain amount of Fe2+ and Fe3+ pyrophosphate heterophases in (b) (phosphide will also be produced above 700°C). However, treatment with Ar+3% H2 cannot improve the purity of the product. The pure phase LiFePO4 cannot be obtained with other Fe(II) salts. In fact, no matter which Fe(II) salt, when the temperature is higher than 500℃, Both will generate Fe(Ⅲ) crystal phase. LiFePO4 prepared by the co-precipitation method is generally in an inert atmosphere, adding LiOH to a mixture of Fe(II) salt and phosphoric acid, washing and drying the precipitate. There are also obvious miscellaneous peaks in Figure (c). It can be seen that the solid phase method is not suitable for preparing pure phase LiFePO4.
Most cathode materials can be prepared by hydrothermal method. For example, common LiCoO2, LiNiO2, LiMn2O4, LiV3O8, LiMnO2, LNiVO3, etc.
It is worth noting that the temperature, pressure, sample processing time and the composition of the solution, acidity and alkalinity, and the type of precursor used in the hydrothermal synthesis process affect the size, form, composition of the system, and whether the product particles are generated. Pure equality has a big impact. In addition, if the synthesized oxide cathode material has a higher solubility than the corresponding hydroxide under high temperature and pressure, it cannot be synthesized by hydrothermal method..
In summary, it can be seen that under mild hydrothermal conditions, on the one hand, chemical reactions that are difficult to carry out in a solution at room temperature and pressure can be carried out smoothly under high temperature and high pressure, and on the other hand, it can be crystallized to obtain a specific valence. , Special configuration, balanced defect crystals. Compared with the solid phase method, it generally only includes the three steps of "raw material preparation hydrothermal reaction, filtration and washing". Compared with the solid phase method and the sol-gel method, the process is simple and the synthetic product purity is higher. There are promising ways.
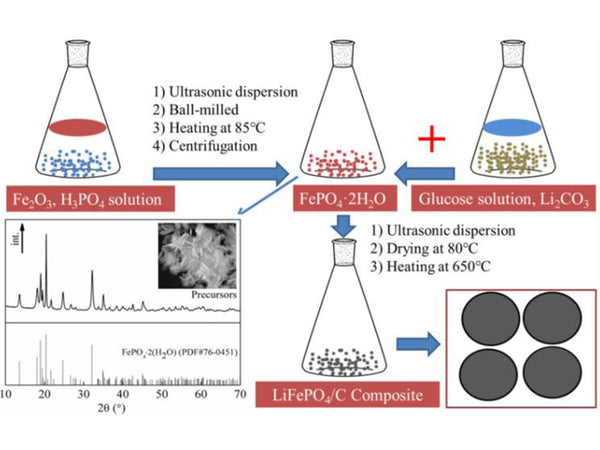
Figure 2 Preparation of Lithium Iron Phosphate LiFePO4