Main content:
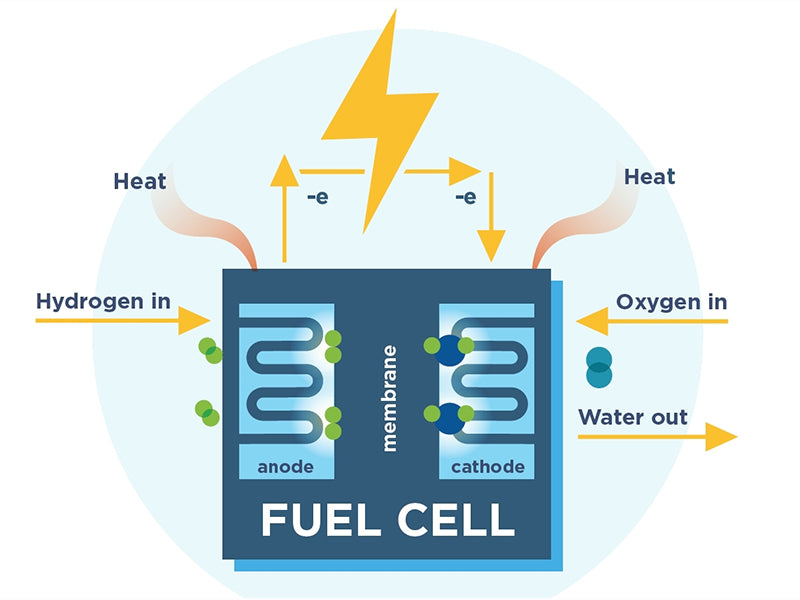
A fuel cell is a system that converts the chemical energy of fuel into electrical and thermal energy.
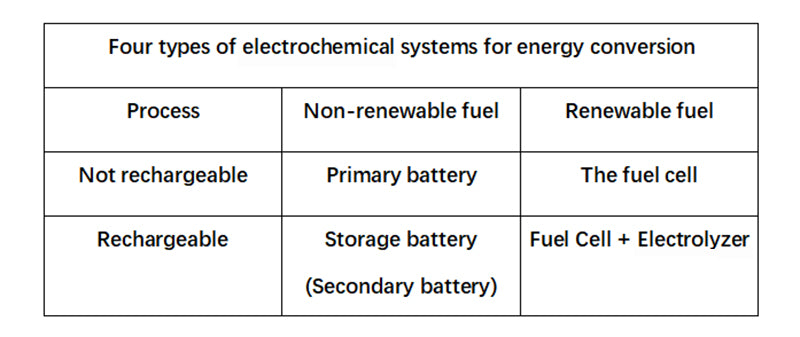
The continuously replaceable chemical reagents are called "fuels" or "combustors," depending on their role in the reaction system, as we'll see later. In this sense, therefore, a fuel cell is a primary cell in which the reactants can be regenerated during use. A fuel cell only reacts in one direction, converting chemical energy into electrical energy, while a "fuel accumulator" can react in both directions. Technically, the latter consists of two units that can operate independently, a fuel cell and an electrolyzer (collectively, the units that convert electrical energy into chemical energy). The diagram below lists four types of devices formed according to the principles of rechargeability and renewability.
Any non-pure electric energy storage process has a two-way conversion process of energy: one direction is to obtain the energy that can be stored, and the other direction is to return it in the form of electricity. A "fuel battery" connects the fuel cell and the electrolyzer together, which is a chemical form of electrical energy storage system.
1.Proton exchange membrane fuel cell
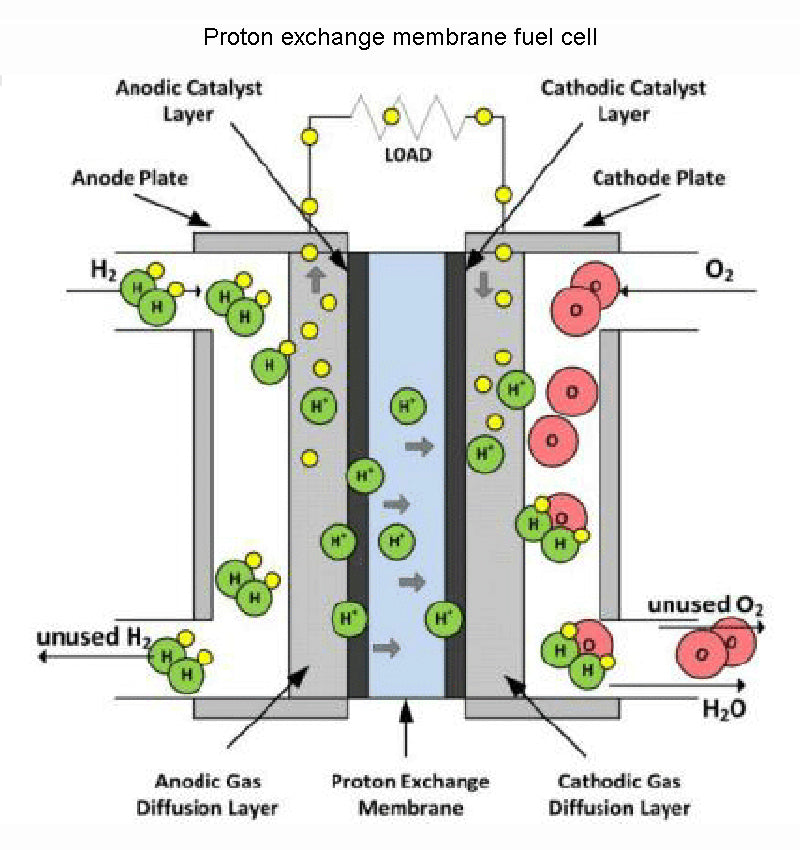
We already know from the previous analysis that the core element of the fuel cell (PEMFC) reaction is the separation of fuel and combustion agent, and the separator must be a poor conductor of reactants and electrons, but must allow protons to pass freely. The problem now is that no such material exists, at least not strong enough to make fuel cell work properly.
This need to be extremely selective for certain ions, yet impermeable to other species participating in the reaction, means that there are very few types of special materials worth investigating. If we ignore factors such as economy and life for a while, this material should at least have good mechanical properties to resist the unavoidable pressure difference, good temperature characteristics consistent with the battery cell, and sufficient resistance to chemical corrosion by reactants. Capability, these are the main criteria for material selection for fuel cell.
In fuel cell, the best separators with high proton conductivity are polymer membranes. The best (and most expensive, €400/m2) product of this type right now is the Nafion membrane exclusively manufactured by DuPont. Several companies are now able to produce proton exchange membrane fuel cell, but the price is high and the lifespan is still very limited (the industry's ideal performance energy level for such fuel cells is expected to be below 50 euros/kW h, to From 2010 to 2015, the running time of more than 5000h can be provided). In order to obtain sufficient proton conductivity in fuel cell, the operating temperature of the membrane should be controlled at 50-80 °C. Better proton conductivity can be achieved at higher temperatures, but at higher temperatures, the membrane's mechanical or chemical properties quickly become insufficient. Using this type of fuel cell as a power source, power supply from 1W to a maximum of about 100kW can be achieved. The on-board systems of vehicles and the power supply of various mobile devices are the main application targets of such fuel cells.
Since it is very difficult to produce such a separator that can effectively conduct protons, it also promotes the research of other kinds of ionic conductors to ensure the controllability of the fuel cell combustion process.
2.Solid oxide fuel cells
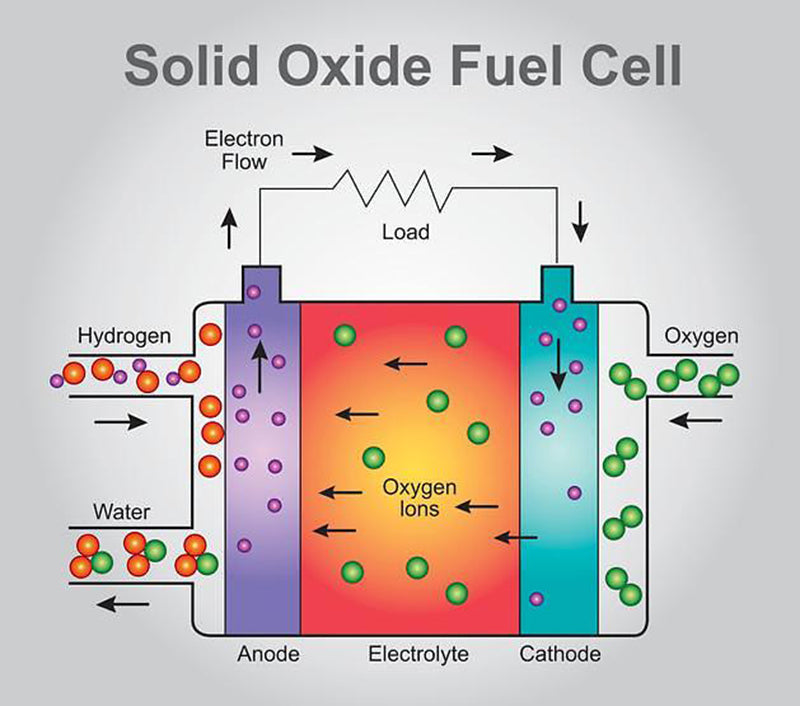
When we analyzed the electrochemical reactions of water production in detail, two partial reactions were mentioned: the oxidation of hydrogen and the reduction of oxygen. From this, it can be seen that the formation reaction of water is
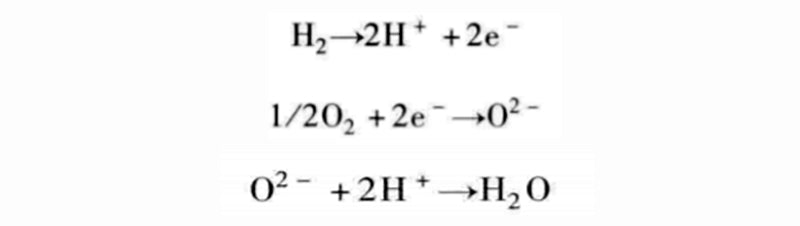
There are many different combinations possible, so the reaction equations used in PEM fuel cells are not the only means of controlling the combustion process.
For example, if you combine the above reaction equations, you get:
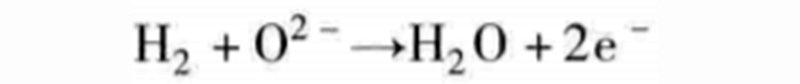
This time the oxygen ions O2 - need to pass through the separator, from the cathode side plenum to the anode side plenum. Therefore, water is generated on the anode side (hydrogen gas), which is distinctly different from PEM fuel cells. The single cell, which relies on the exchange of oxygen ions, is named solid oxide fuel cell (SOFC) based on the chemistry of its separator.
In solid oxide fuel cells, the materials that can conduct oxygen ions are metal oxides (ceramic materials) of these specific materials, which not only have suitable ionic conductivity, but also need to be sufficiently stable. Another very important feature is that it must be able to work reliably in a high temperature environment of 800~1000℃.
Several ceramic materials are recommended for use in solid oxide fuel cells, but dissecting these materials in detail will take a long time because some of them are very complex, mixed with various metal oxides, and have a variety of stable phases. . We can cite beta-phase alumina, epidote, lanthanum strontium, molybdenum oxide, vanadium oxide, yttrium zirconium mixed oxide, and the like. The main difference between these materials is their ionic conductivity, the most important of which is their stability and their performance at the lowest achievable operating temperature.
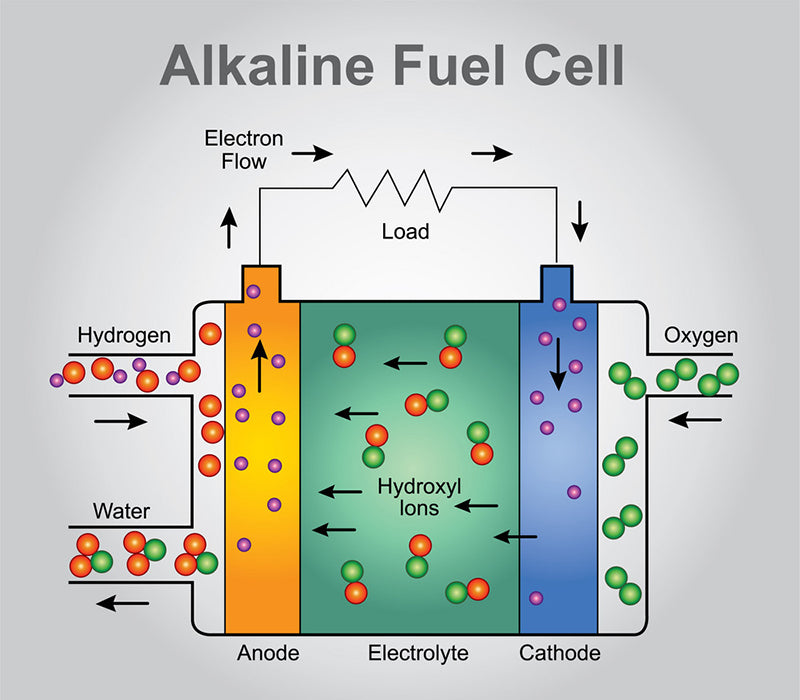
3.Alkaline fuel cells
Although it appears that we have achieved a combination of one reaction with the other two, the reactions discussed here are not elementary reactions because they all involve multiple pairings. For example, instead of adding two protons at the same time, the intermediate reactant of hydroxide ion OH- can be decomposed into the following two steps:
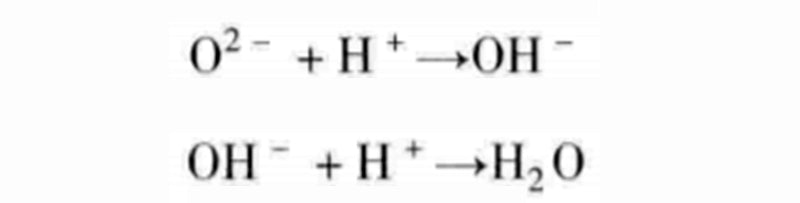
With water supplied to the oxygen chamber, hydroxide ions can be exchanged between the two gas chambers, which will initiate the following two half-reactions:
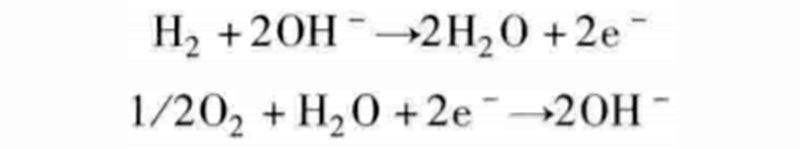
Such fuel cells that exchange hydroxide ions are called alkaline fuel cells (AFC). Its name comes from the fact that the battery needs to operate in an alkaline environment, generally using potassium hydroxide KOH to supply hydroxide ions to the anode gas chamber.
In contrast to the previous two types of fuel cells, alkaline fuel cells have a tube at the bottom that transfers half of the water produced by the oxidation of hydrogen to the cathode plenum for the production of hydroxide ions.
Alkaline fuel cell separators should be good conductors of anions, most of which are polymers that do this. However, unlike proton exchange membranes used in proton exchange membrane fuel cells, anion exchange membranes are more selective and easier to fabricate than Nafion membranes. For alkaline fuel cells, this is definitely the advantage of its industrialization development. However, alkaline fuel cells have a fatal weakness. The alkaline environment is extremely corrosive to electrode metals, so the life of alkaline fuel cells is limited.
4.Comparison of different types of fuel cells
The figure below visually explains the operation of the three fuel cells we have mentioned.
There are not only these three types of fuel cells, especially when different reactants are added to the other gas chamber of hydrogen, which can be expanded into many different combinations to form other types of fuel cells. The table below does not list all possibilities, but only the three types we have analyzed, Molten Carbonate Fuel Cell (MCFC) and Phosphoric Acid Fuel Cell (PAFC). In the application target column, only the most conventional application form is given, but it is by no means limited to this.
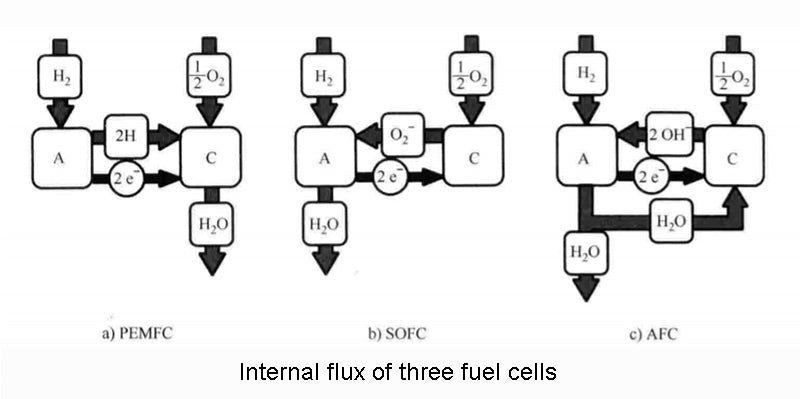
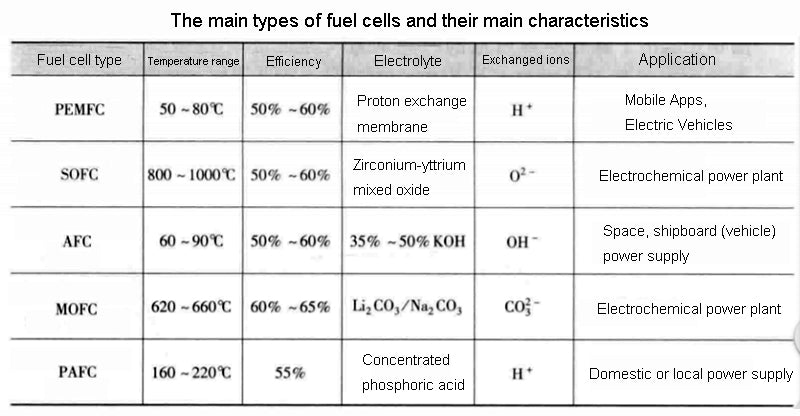
The relevant data of the fuel cell in the above figure are only statistics for hydrogen as the fuel. It should be noted here that other fuels are also feasible, and hydrocarbons can be used as fuel for fuel cells. For example, methanol can directly replace hydrogen as a fuel according to the following chemical reaction equation:
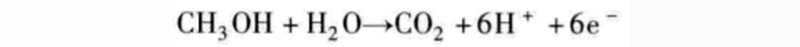
Since protons are ions that are exchanged, a proton exchange membrane like Nafion is required. This fuel cell, called a direct methanol fuel cell (DMFC), has the advantage of operating at room temperature, but the disadvantage of emitting carbon dioxide. Such fuel cells are particularly suitable for mobile applications due to the ease of replenishment of this liquid fuel.
Other fuels include methane CH4, which is converted into hydrogen through a so-called reforming process in the high-temperature region where solid oxide fuel cell operate, so it can be used directly in solid oxide fuel cell just like hydrogen. This reforming process is combined with the fuel cell, giving the solid oxide fuel cell the capability of "internal continuous reforming". There are other types of fuels, which cannot be produced by battery cells because they cannot be reversely reacted, and are not suitable for use as energy storage media, so they are not listed here.
In the future, fuel cells have bright prospects. Like lithium-ion batteries, they have many performance advantages, such as environmental protection, long service life, etc., which solves the pain points of traditional batteries in application.