
Main content:
This paper introduces other indirect hydrogen storage modes or hydrogen storage systems, such as non-reversible hydrogen storage systems, chemical mixture hydrogen storage systems, and chemical-physical hybrid hydrogen storage modes.
1. Indirect hydrogen storage mode using borate
Borate or borohydride (LiBH4, NaBH4, …, Ca(BH4)2) has a molecular structure similar to that of aluminohydrides, and since these materials have two forms that release regassing, we classify them separately. It is a heat release, but the temperature at which the hydrogen release process of this material occurs (the minimum temperature is greater than 400 ° C) has been proved to be too high to be practical. The other is the hydrolysis of hydrogen reaction. As early as the early 2000s, automakers already recognized the viability of this option in experiments to supply hydrogen to fuel cells. In addition, the idea of using integrated containers similar to "disposable" cartridges to supply hydrogen to mobile systems has also been proposed.
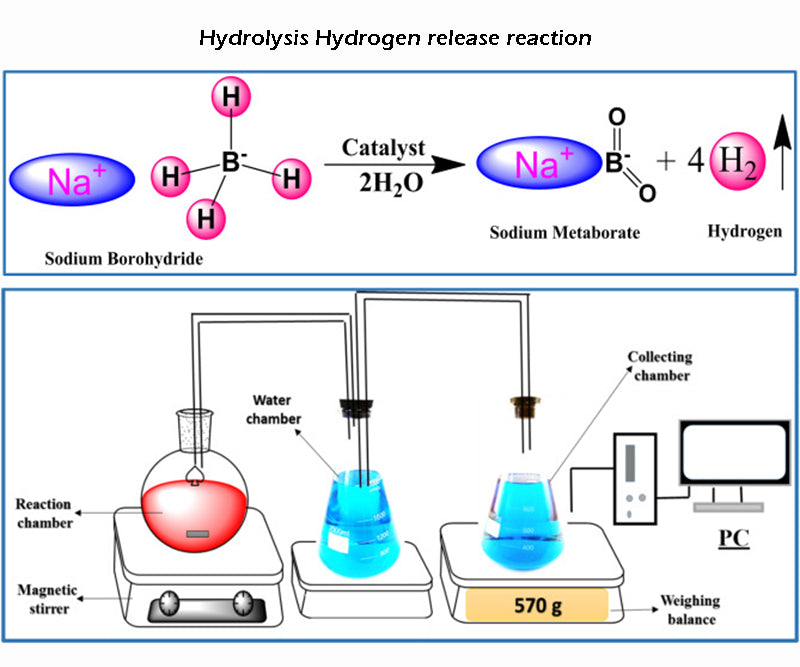
According to the second hydrogen release mode, the sodium compound NaBH4 (theoretical storage density is 15.6%) is the only reaction kinetics for this type of reaction process:
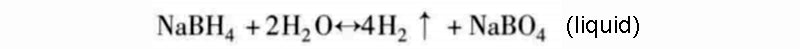
As the reaction is exothermic, the hydrolysis of hydrogen can in principle occur (in part) without any additional energy. But in terms of reaction kinetics, the reaction needs to be between 140 and 180 °C to start. Using saturated borax solution can easily control the reaction rate of the hydrolysis complex reaction, and this process is also stable; and according to the above reaction equation, adding a catalyst can theoretically promote the decomposition of borate. In fact, the borate hydrolysis reaction is incomplete, and the final reaction product is not in the form of oxide, but a more stable hydroxide. Therefore, the actual amount of hydrogen released in this hydrogen storage mode is reduced to about half. Only when the concentration of sodium borohydride solution is above 5%, the above-mentioned hydrolysis of hydrogen reaction will occur. For high-volume production of borate, care must be taken not to generate briquettes during chemical regeneration, as it is highly toxic.
2. Indirect hydrogen storage mode using a mixture of salts and hydrides
In order to reduce the overall temperature of the borate hydrogen release reaction, mixing borate with low-temperature hydrides such as MgH2 and CeH2 is also a solution. According to the existing analysis, the reaction temperature is around 400°C, but the reaction needs to be carried out in multiple steps, the process is complicated, it is difficult to control, and the reaction cannot be completed. Therefore, as far as the current situation is concerned, the results are not very optimistic.
3. Hybrid hydrogen storage mode

Among the above-mentioned compounds, the hydrogen storage mode of the hydrogen storage mode has the highest hydrogen storage density. The BCC alloy uses a medium pressure (200-300 bar) pressure vessel, and the hydrogen storage limit density of the saturated hydrogen storage mode is close to 3.7%. When using high-pressure hydrogen storage mode, "inactive" parts must remain between grains, and the intermetallic compounds in the hydrogen storage mode container cannot be over-compressed, because during the hydrogen production process, the stress caused by the increase in volume will Damage the container shell. This hybrid hydrogen storage model can achieve a hydrogen storage density of 6%, which may be feasible for some automakers. However, the mechanical and thermal stresses generated by the cycle process of multiple hydrogen storage modes still need further verification on the performance of the storage composite alloy, the mechanical properties of the storage-recovery container and its built-in hydride.
Read more: What is pressure hydrogen storage?