
There are two types of natural graphite: amorphous graphite and flake graphite. Amorphous graphite, also known as microcrystalline graphite, is composed of non-oriented graphite crystallites. Graphite crystal plane spacing (d002) is 0.336nm, mainly hexagonal crystal planes, the reversible specific capacity is only 260mA·h/g, and the irreversible specific capacity is above 100mA·h/g. The interplanar spacing (d002) of flake graphite is 0.335nm. Its structure has not only hexagonal crystal planes, but also rhomboid crystal planes. The existence of rhombic crystal planes improves the charge and discharge capacity and the reversible ratio The capacity can reach 300~350mA·h/g, and the irreversible specific capacity is less than 50mA·h/g. Figure 1 is a typical charge and discharge curve of graphite. During the first charge, there is a small platform around 0.8V, which corresponds to the reduction of solvent molecules on the graphite surface to form an SEI film. In the second charge, this platform disappears; at 0~0.3V long The voltage plateau corresponds to the process of lithium intercalation in graphite.
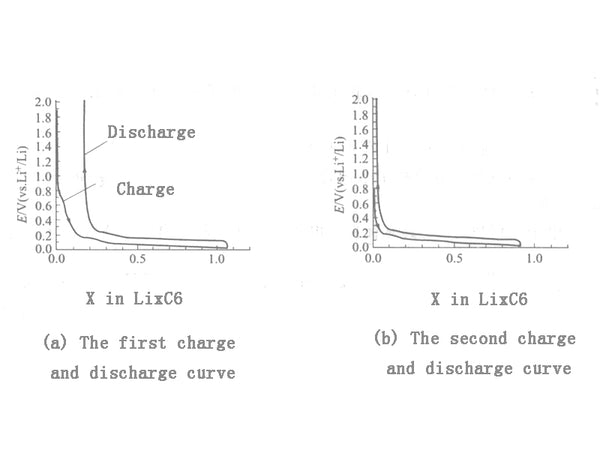
Figure 1 Typical charge and discharge curve of graphite
Before lithium is inserted into the graphite, an SEI film will be formed on the surface of the graphite electrode. The quality of the SEI film directly affects the performance of the electrode. If the membrane is unstable and not dense enough, on the one hand, the electrolyte will continue to decompose; on the other hand, the solvent will be co-embedded, leading to the destruction of the graphite structure. Therefore, the SEI film determines the reversible capacity of graphite and also affects the stability of the graphite anode. Studies have shown that natural graphite in PC, BC, and mixed solvents containing PC or BC, does not form a stable SEI film due to the generation of gas at 1.0V, which leads to the co-embedding of solvent molecules and peeling off the graphite layer. The exfoliation of graphite is caused by the stress generated by the co-inserted solvent molecules or its decomposition products exceeding the attraction of the van der Waals force between the graphite layers, which can significantly increase the graphite layer spacing. The phenomenon of graphite exfoliation mainly depends on how easy it is for solvent molecules to intercalate into the graphite layer and whether there is a stable SEI film. The difficulty of co-embedding of solvent molecules in the graphite layer is related to the structure of graphite itself, such as crystallinity and the structure of solvent molecules. On the one hand, defects in the graphite structure can act as electron acceptors and reduce the Fermi level of carbon materials; on the other hand, certain structural defects can inhibit the movement of graphite flake molecules between each other and inhibit the polar solvent molecules of the electron acceptor. The total insertion.
The structure of solvent molecules obviously affects the degree of exfoliation of graphite. If solvent molecules have "pointed" positions, co-insertion may cause damage to the graphite structure. PC and BC are solvents with such "pointed" positions, so graphite is in them Can not be charged.

Figure 2 Schematic diagram of lithium competition on graphite electrodes
Some factors that affect the electrochemical performance of graphite include particle size and distribution, morphology, orientation, graphitization degree, and preparation conditions of graphite electrodes. Small particles of graphite (about 6μm) have better high-current charge and discharge performance than large particles (about 44μm). When the charge and discharge capacity of small particles of graphite at the rate of C/2 can still reach 80% of the charge and discharge capacity at the rate of C/24; while the charge and discharge of large particles of graphite at the rate of C/2 can only reach the charge and discharge capacity of the rate of C/24 25%. The reason is that, on the one hand, small particles can reduce the current loaded per unit area, which is beneficial to reduce overpotential; on the other hand, the edges of small particles of carbon crystallites can provide more migration channels for lithium ions; at the same time, lithium ions can migrate. The path is short and the diffusion resistance is small. However, the barrier effect between small particles will reduce the liquid phase diffusion rate. On the contrary, although large particles are conducive to the liquid phase diffusion of lithium ions, the solid phase diffusion process of lithium ions in the carbon material becomes relatively difficult. The result of the competition between the two makes the carbon material have the best particle size and distribution.
The orientation of graphite is very important to the high-current performance of the negative electrode, because the diffusion of lithium ions in graphite has a strong directionality, that is, it can only be inserted from the end face perpendicular to the direction of the graphite crystal axis. If the graphite orientation is parallel to the set Fluid, the migration path of lithium ions is longer, resulting in a decrease in the diffusion rate and reducing high-current performance. If the orientation of the graphite sheet is parallel to the current collector, the lithium ions do not need to go through a curved path, and the deintercalation of lithium ions can occur directly, so the diffusion resistance is small, which is conducive to high-current charging and discharging. However, due to the translational nature of graphite flake molecules, most of the graphite flake molecules are stacked in parallel current collectors during coating and extrusion processes, and it is difficult to achieve vertical current collectors.
The various groups present on the graphite surface have a significant effect on the exfoliation of graphite. If there are acidic groups on the surface, peeling will not easily occur.