
What are the layered oxides of molybdenum and vanadium?
1.1 High-valent oxides of vanadium and molybdenum
V2O5 and MoO3 are the first two oxides studied. One molybdenum in the compound MoO3 easily reacts with about 0.5 lithium, but the reaction rate is slow. When the MoO3 solid is added to n-butyllithium, the rate of this reaction can be easily measured by the rate of temperature rise. Figure 1 shows the reaction heat of the three cathode materials TiS2, V2O5 and MoO3. The higher the temperature, the greater the power of the material.

Figure 1 Reaction heat of three cathode materials Tis2, V205 and MoO3
V2O5 is a layered structure, the vanadium-oxygen bond between the layers is very weak, and the reaction with lithium is through the intercalation mechanism: χLi+V2O5=LiχV2O5. The structural characteristics of V2O5 are quite complicated when lithium is intercalated. Initially, lithium is intercalated into the structure, and α phase (χ<0.01) is formed, and then ε phase (0.35<χ<0.7) is formed, and the middle layer of ε phase is more folded. At χ=1, one of the two layers has shifted, resulting in the formation of δ phase. However, when more than one lithium is charged, a major structural change occurs, and the γ phase exists in the range of 0<χ<2. In the α-phase, ε-phase and δ-phase, the constituent V2O5 structure is arranged in a square cone, with the vertices arranged on the upper, upper and lower sides. The difference is that in the highly folded γ phase, these vertices are arranged up, down, up, and down. When more lithium is inserted, a sodium chloride structure is formed, and this compound is called ψ-Li3V2O5 phase. When the small phase in a single solid solution phase is electrochemically cycled, the final release of lithium is about 4V, which clearly shows that this phase is the same as the initial V2O5 phase (open circuit voltage is 3.5V)
Is different (Figure 2). As shown in Figure 3, the 4 substance has a tetrahedral structure, after a long period of time, it becomes a simple sodium chloride structure, the chemical formula is Li0.6V0.4O, and its unit cell parameter a=0.41nm.
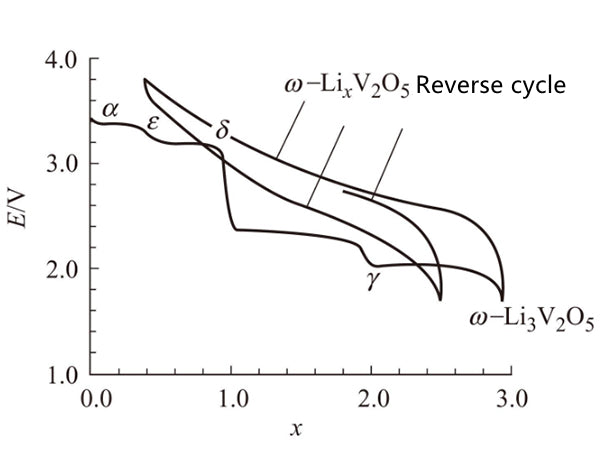
Figure 2 Discharge performance of V205 compound
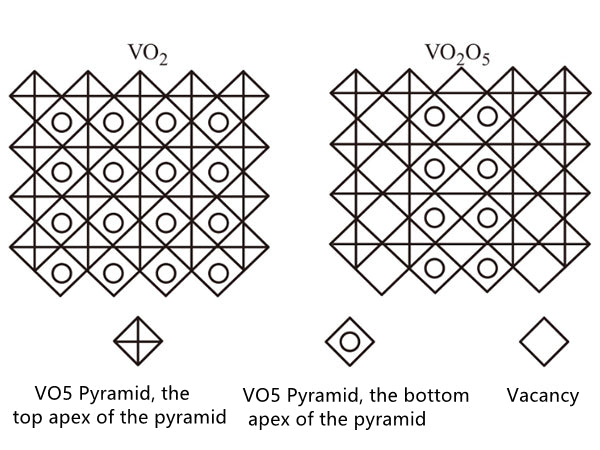
Figure 3 Structure diagram of V205
1.2 Mixed valence vanadium oxide
Partially reduced vanadium oxides such as V6O13 have good electrochemical performance. In the preparation of lithium vanadium oxide compounds, the Li:V molar ratio is 1 to combine with lithium, and the V:O molar ratio is in the reaction of vanadium oxide and lithium. The capacity control aspect plays a major role. This type of compound is a staggered single and double layer structure in which the single layer of vanadium oxide is a deformed VO6 octahedron. There are many positions for the insertion of lithium ions. Once these positions are filled, the relevant steps shown on the discharge curve will be carried out. Studies have shown that the compound’s lattice first expands along the c-axis and then along the b-axis.
The vanadium oxide LiV3O8 is a layered structure composed of octahedrons and triangular bipyramid, which can increase the insertion of lithium ions like other layered structures. Similarly, the preparation method has a great influence on the electrochemical performance of LiV3O8; in the low current range (6~200pA/cm2), the capacity of LiV3O8 amorphous material with the same Li/V molecular ratio increases from 2 to 3 per mole. ~4 and above.
1.3 Double-layer structure: dry gel, δ-vanadium oxide and nanotubes
Vanadium oxide can be obtained by acidifying sodium vanadate solution. For example, sodium vanadate exchanges acidic ions, and the molecular structure of the dried orange gel is HχV2O5·nH2O. Under vacuum or moderate heating conditions, based on its high cation exchange capacity, after removing about 1.1 mol of water, it becomes H0.3V2O5·0.5H2O, and the inner layer spacing is about 0.88nm. In the presence of 1.8H2O, The distance is increased to 1.15 nm, and protons and water are exchanged by lithium ions and polar solvents. In addition, vanadium oxide xerogels can also be prepared by treating V2O5 with hydrogen peroxide. The vanadium pentoxide obtained by this method contains two layers of vanadium oxide flakes. Under the action of the external vanadium-oxygen bond, the tetragonal cone formed in the crystalline V2O5 structure becomes a twisted octahedral structure. The active product HyV2O5·nH2O·carbon prepared by the aerogel process under supercritical drying conditions in the presence of CO2 and acetone has good electrochemical performance, and the mass ratio of carbon is about 3.9%; after drying, The lattice spacing can reach 1.25nm, and the intermediate electromotive force that reacts with lithium on a single sustainable discharge curve is about 3.1V, and 4.1 lithium can be combined with every 2.8V, as shown in Figure 4 Figure 5, its capacity is much higher than that of crystals. State of V2O5.
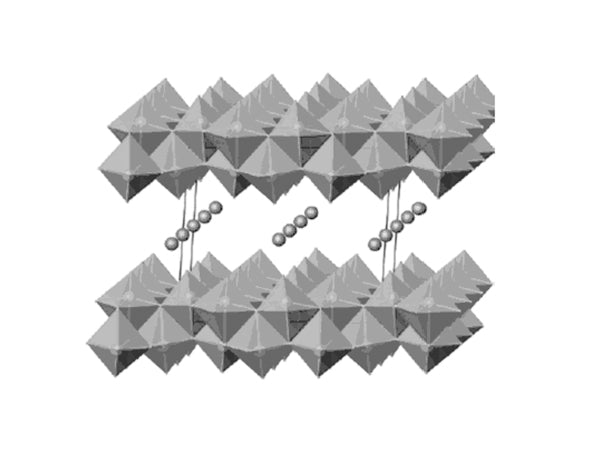
Figure 4 Structure diagram of double-layer vanadium oxide made by aerogel
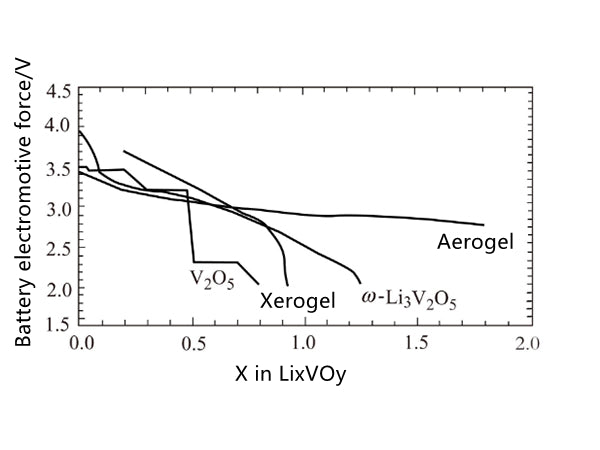
Figure 5 The electrochemical properties of V2O5 with different crystalline forms
The vanadium oxide double-layer structure in the xerogel forms a double-sided layer. Vanadium occupies the distorted VO6 octahedral position. The oxide exhibits excellent electrochemical capacity, which can exceed 200mA·h/g in some cases, as shown in Figure 6 shown. However, the specific capacity of the compound is still limited to a certain extent. Therefore, more vanadium oxide nanotubes are synthesized and utilized. The vanadium oxide nanotubes contain a double-layer vanadium oxide structure and exhibit interesting but complicated electrochemical behavior. Under certain conditions, the capacity increases during the cycle, and the electrochemical behavior of the compound formed by replacing part of the vanadium ions with manganese ions is shown in Figure 6.
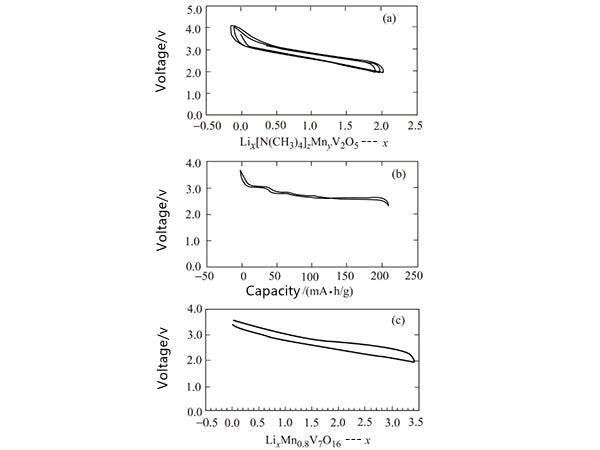
Figure 6 Electrochemical performance of δ-MNyV2O5 (a), δ-NH4V4O10 (b) and manganese vanadium oxide (c)