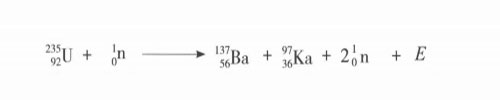main content:
- 1 Atomic mass loss phenomenon
- 2 Nuclear fission and nuclear fusion
- 3 what is the atomic bomb
1 Atomic mass loss phenomenon
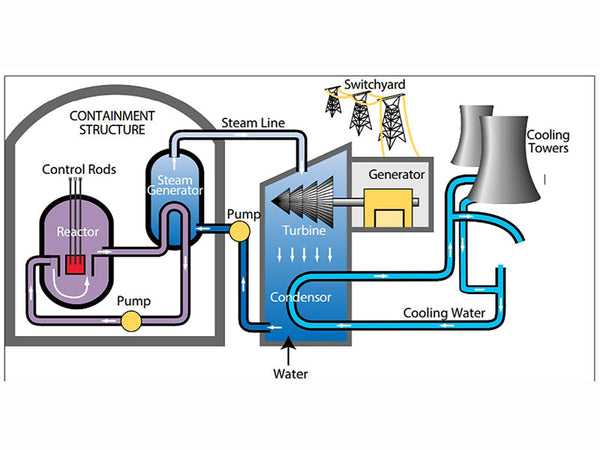
The molecule of a substance is composed of atoms, which are composed of positively charged nuclei and negatively charged electrons. The nucleus is composed of protons and neutrons (collectively called "nucleons").
Schematic diagram of atomic structure
When atoms are combined or separated, the position and movement of electrons are changed, and the energy released is called chemical energy. For example, the combination of carbon atoms in fuel and oxygen atoms in the air will release a certain amount of chemical energy. Chemical energy does not "distort" the nucleus. If you can manage to combine or split the nucleus, you can also release energy. This energy released by the change of the nucleus is commonly known as "atomic energy", or nuclear energy for short, or "nuclear energy" for short. The protons in the nucleus are all positively charged. When they are squeezed into a space with a very small diameter (about 1×10-12 cm), their repulsive force is very huge. However, not only did the proton not fly away, but on the contrary, it was tightly bound to the uncharged neutron, which shows that there is still a much stronger bond between the nucleus than the electromagnetic force (such as the force between the nucleus and the electron). Force, this force is called "nuclear force". Scientists have discovered that the mass of the nucleus before and after the split or union of the atomic nucleus is very different. For example, the total mass of the fissioned uranium-235 fragments (nucleus with fewer nuclei and neutrons, etc.) is a little less than before fission, about one-thousandth, indicating that a mass loss has occurred after fission. For another example, the helium nucleus is composed of 4 nucleons (2 protons and 2 neutrons), and the total mass is 4.002663 atomic mass units. However, if the masses of the 4 nucleons are added together, it should be 4032980 atomic mass units. , Which shows that mass loss has also occurred after the nucleus is combined.
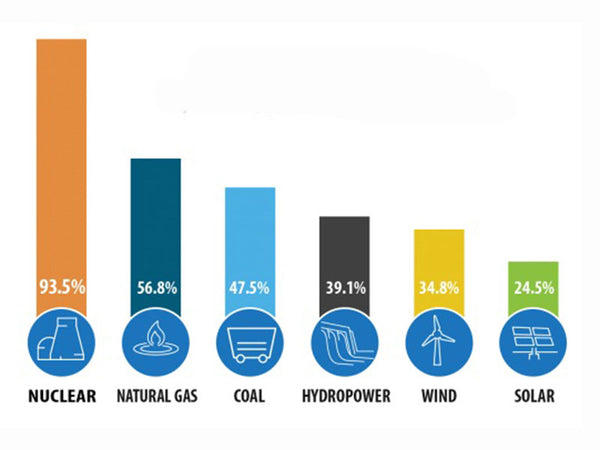
What causes the quality loss phenomenon? Where does the quality of losses go? The phenomenon of mass loss is caused by the aforementioned "nuclear force" that exists between nuclei. In other words, the lost mass is transformed into nuclear power. When the nuclear fission or fusion occurs, it is released in the form of energy. This energy is the nuclear energy we want to seek and use. So, how big is this nuclear energy? According to Einstein's mass-energy conversion formula E=mc2 (E is the energy m is the mass, c is the speed of light constant), it can be known that in nuclear fission or fusion, although the "lost" mass is only a little bit, it is multiplied by the absolute value of C2. Value, the energy gained is amazing! The nuclear energy released by fission of 1 kilogram of uranium-235 is equivalent to the chemical energy produced by the combustion of 2,700 tons of standard coal. The energy produced by the same mass of uranium and coal differs by 2.7 million times. If it is a nuclear fusion reaction, the energy produced is much larger.
Comparison of the calorific value of nuclear fuel and coal
2 Nuclear fission and nuclear fusion
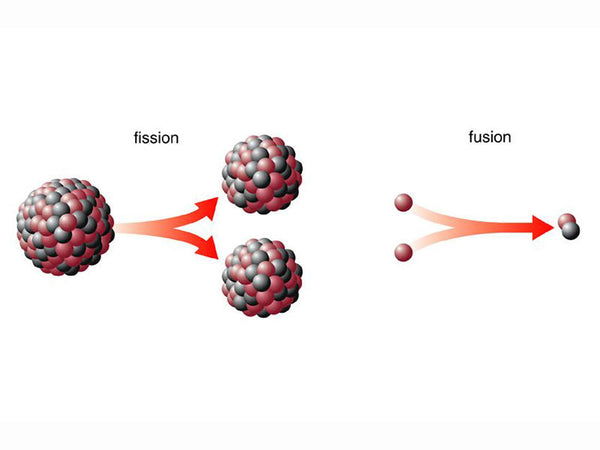
The energy released when a nucleus is combined into a nucleus is called the total binding energy of the nucleus. Due to the different degree of tightness of the bonding of various nuclei and the different number of nuclei in the nucleus, the total binding energy is also different. Therefore, there are two different ways of using nuclear energy-nuclear fission and nuclear fusion.
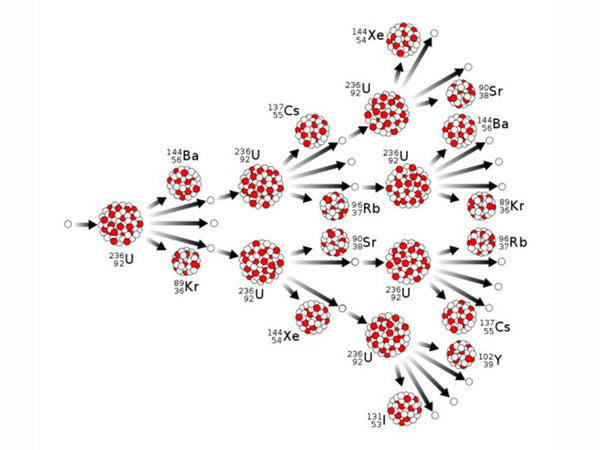
Nuclear fission is the splitting of a "heavy nucleus" (referring to a nucleus with a large number of nucleons) with a smaller average total binding energy into two or more medium-mass nuclei with a larger average total binding energy, simultaneously releasing nuclear energy. Heavy nuclear fission has two forms: "spontaneous fission" and "induced fission". Spontaneous fission is caused by the instability of the heavy nucleus itself, with a long half-life and unable to use its energy; induced fission is the heavy nucleus being affected by other particles, especially The bombardment of neutrons splits into two lighter nuclei with different masses, releasing energy and neutrons at the same time. This energy is the nuclear energy that people can use. Induced fission is a "chain reaction", like the chain of a chain, it "reproduces" ring by ring. Induced fission chain reactions are divided into two types: uncontrollable and artificially controlled. Only artificially controlled chain reactions are needed for the peaceful use of nuclear energy. Nuclear fission can only occur in the range of heavy nuclei (above 200 atomic mass units), and uranium-235 is the only nuclide prone to nuclear fission that exists in nature. The typical fission reaction of uranium-235 is shown in the following formula. This is only one type of uranium fission reaction. In fact, there may be 70 or 80 types of uranium fission fragments, all of which are radioactive. Together with their progeny, there are about 200 kinds of fragments, collectively called fission products.
Uncontrolled chain reaction diagram
|
tips: chain reaction
|
Nuclear fusion is the aggregation of light nuclei (such as deuterium and tritium) with a small average total binding energy into an atomic nucleus with a large average total binding energy, while releasing huge amounts of energy. Due to the strong electrostatic repulsion between atomic nuclei, only at ultra-high temperatures of tens of millions of degrees Celsius, light nuclei have enough kinetic energy to overcome the electrostatic repulsion and fusion. Therefore, ultra-high temperature is an external condition necessary for nuclear fusion to occur. , So it is called "thermonuclear reaction". The electrostatic repulsion of an atomic nucleus is proportional to the charge it carries. Therefore, the lower the atomic number and the lower the number of protons, the lower the kinetic energy (temperature) required for the fusion of light nuclei, so only some lighter nuclei (such as hydrogen, deuterium) , Tritium, helium, lithium, etc.), are prone to nuclear fusion. In other words, nuclear fusion only occurs in the range of light nuclei (nuclei with a few to a dozen atomic mass units). The most common nuclear fusion reaction is a process in which deuterium and tritium react to generate helium and neutrons and release nuclear energy (E) at the same time. The reaction formula is as follows:
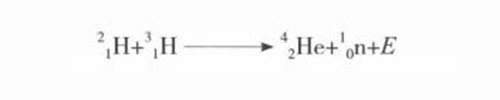
Atomic reaction formula
Nuclear fusion requires very high temperature conditions. Currently, it can only be achieved in the explosion of a hydrogen bomb and the collision of high-energy particles produced by an accelerator. The former is an uncontrollable nuclear reaction, and the latter is difficult to sustainably use. Therefore, to achieve a controllable and sustainable nuclear fusion reaction and provide humans with nuclear fusion energy, another way is still to be found.
3 what happened to the atomic bomb

The use of nuclear energy to produce electricity, commonly known as nuclear power. When talking about nuclear power and nuclear power plants, many people immediately think of atomic bombs and hydrogen bombs (collectively referred to as nuclear bombs). In fact, nuclear power and nuclear bombs are not the same thing. Nuclear power plants are absolutely impossible to have a nuclear explosion like an atomic bomb. This is not because safety measures can prevent it, but because it is impossible based on scientific principles. It is completely unnecessary to worry about a nuclear explosion.
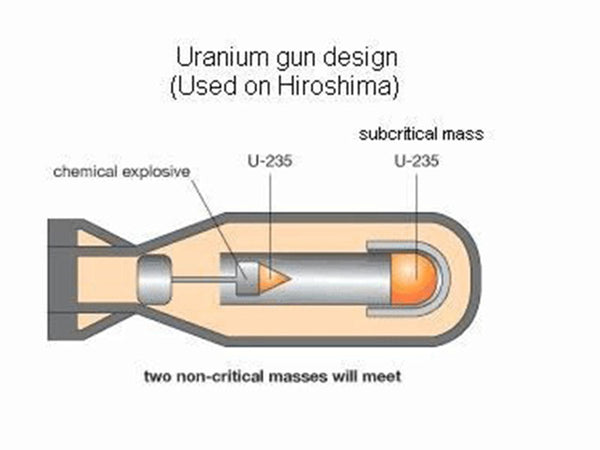
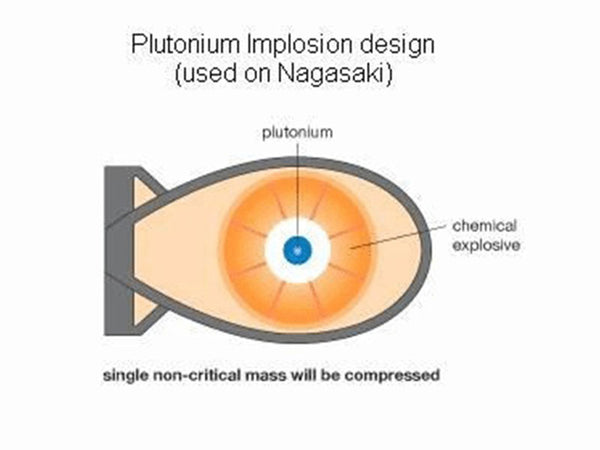
The fission of uranium-235 is easy to proceed in a chain and automatically under certain conditions. There are two “certain conditions”: one is to increase the purity of the “uranium pile” (ie, the pile composed of uranium-235) and reduce The content of impurities. Because impurities, including uranium-238, will intercept most of the neutrons in the absorption reaction, interrupting the chain reaction. Second, the volume of the uranium reactor must be large enough to prevent neutrons from flying out of the reactor, otherwise the chain reaction will be interrupted. The neutrons produced by each fission of the uranium nucleus are fast neutrons, which are easy to fly out of the reactor. This "sufficiently large" volume at least reaches the so-called "critical volume", that is, the volume that can make the chain reaction continue automatically. For highly enriched uranium-235 (content above 93%), the critical volume diameter is about 4.8 cm, and the corresponding mass is called "critical mass", which is about 1 kg. Under these two conditions, how does the chain reaction proceed? As mentioned earlier, each uranium nuclear fission produces 2~3 new neutrons, and each new neutron can fission other uranium nuclei. This chain reaction proceeds very quickly, and It is automatic and uncontrolled. The entire uranium pile is fissioned in far less than 1 second (10-7s), and the energy is released at the same time, that is, a nuclear explosion occurs. This is the principle of the atomic bomb. In the production, more than 1 kilogram of highly enriched uranium-235 is made into two pieces, each smaller than the critical volume. When using it, first let the ordinary TNT explosive explode, generating pressure to push the atomic explosive (uranium block) together, and the combined total volume exceeds the critical volume of the uranium block, so the atomic bomb explodes. The atomic bomb made of 1 kilogram of uranium-235 has a power equivalent to about 2 Ten thousand tons of TNT explosives are called 20,000-ton nuclear bombs (that is, nuclear bombs are classified with TNT explosives as equivalent). The first atomic bomb dropped by the United States on Hiroshima, Japan on August 6, 1945 was a 20,000-ton nuclear bomb.
Schematic diagram of the atomic bomb structure
Nuclear power plants also need to carry out nuclear fission chain reactions through "nuclear reactors" in order to continuously deliver nuclear energy for use. However, this kind of chain reaction is controlled, so that even if the chain reaction can continue, it is not excessively violent to cause safety accidents. Enriched uranium-235 is also used as the "nuclear fuel" of nuclear power plants in order to play the role of high power plants. However, this kind of enrichment does not need to be too demanding. Uranium-235 can account for about 3% of the uranium reactor, and the rest is uranium-238 isotope. It can be seen that this kind of uranium reactor will never reach the conditions for a highly enriched uranium nuclear explosion. Even if the chain reaction is out of control, causing serious accidents such as overheating and melting of the core, a nuclear explosion will not occur. This is the absoluteness that nuclear power plants will not have a nuclear explosion, which shows that it is not the same thing as an atomic bomb. Some people like to say that, just as alcohol and high-grade liquor can be burned on fire, but alcoholic beer cannot be burned, it is quite appropriate.
Another issue is quite noticeable. In the reactor of a nuclear power plant, the main component of the fuel pellet is the uranium-238 isotope, which cannot be directly fissioned by neutrons, but is easily absorbed by it. After uranium-238 absorbs neutrons, it undergoes two "beta decays" and becomes the 94th element plutonium-239, which is present in spent fuel (ie, "spent fuel"). It is another nuclear fission fuel that can also be used to produce atomic bombs. Therefore, the International Atomic Energy Agency (IAEA) has the supervisory function of the "Nuclear Non-Proliferation Treaty" and is very sensitive to the issue of nuclear power plants. As long as plutonium-239 is extracted from “spent fuel”, it may be used to produce nuclear weapons. Of course, plutonium-239 can also be produced by a special reactor. On August 9, 1945, the atomic bomb dropped on Nagasaki, Japan was made of plutonium-239, which is twice the equivalent of the atomic bomb that fell on Hiroshima.
Another type of nuclear bomb is the hydrogen bomb. Simply put, the hydrogen bomb is detonated by an atomic bomb. The "nuclear explosive" used in hydrogen bombs is also deuterium and tritium in principle, but the actual bomb is lithium deuteride. Lithium produces tritium during the explosion of the bomb. Under the ultra-high temperature generated by the atomic bomb explosion, the fusion materials deuterium and tritium instantly undergo nuclear fusion, releasing energy at the same time, so they are extremely powerful. The structure of the hydrogen bomb is shown in the figure. Hydrogen bombs are not limited by the "critical volume" like atomic bombs, so they can be made very large. Its power level varies according to the amount of nuclear material it contains, and the largest can reach more than 10 million tons of TNT equivalent.
Schematic diagram of hydrogen bomb structure
Controllable nuclear fusion also requires extremely high temperature conditions to utilize nuclear energy, but nuclear fuel uses "plasma" to continuously generate micro-nuclear fusion, gradually releasing energy and transmitting it in time. The principle is also different from hydrogen bombs, and of course, nuclear fuel does not occur. explode.