main content:
The battery is usually composed of three parts: positive electrode, negative electrode and electrolyte. The chemical system of the battery determines the electrochemical reaction of the battery, and also determines the theoretical voltage, theoretical specific capacity and theoretical specific energy of the battery. The development and research of lithium-ion batteries mainly focus on related active materials, which mainly include cathode materials, anode materials and electrolyte systems of lithium-ion batteries. The performance and preparation process of battery constituent materials largely determine the performance of lithium-ion batteries, especially the cathode and anode materials are particularly important. Both the positive electrode material and the negative electrode material should have the following characteristics:
① There is a large reversible Gibbs energy in the reaction with lithium ions, which can not only reduce the energy loss caused by polarization, but also ensure a high electrochemical capacity;
②Lithium ions have a large diffusion coefficient in it, which can not only reduce the energy loss caused by polarization, but also ensure faster charge and discharge;
③ The structural material has good stability in the range of charge and discharge voltage, which can ensure the life cycle of the lithium-ion battery;
④ The discharge voltage stability of the material is good, which is beneficial to the wide application of lithium-ion batteries.
1. Cathode materials for lithium-ion batteries
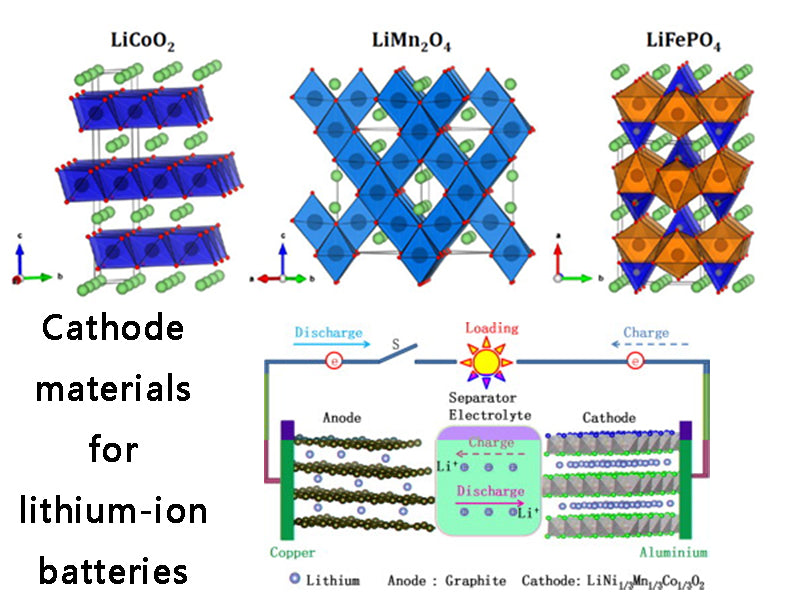
The most widely used cathode materials for lithium-ion batteries are lithium-intercalating transition metal oxides and lithium-intercalating organic sulfides and their polymers.
1) Lithium intercalation transition metal oxides
Lithium-intercalated transition metal oxides are intercalated compounds formed by lithium and transition metals, and are currently the main cathode materials used in lithium-ion batteries. The main transition metal oxides currently used are LiCoO2, LiNiO2, LiVO2 and LixMnyOz and their dopants. Since LiCoO2 was first used by Sony and entered the market in 1990, its discharge capacity, reversibility, charge-discharge efficiency, and voltage stability have been very good, and it has been in a monopoly position. However, it is expensive and toxic, and its capacity is generally limited to 125A·h/kg in order to ensure no loss of irreversible capacity and a certain polarization voltage. LiNiO2 is the most studied layered compound after LiCoO2. Nickel has similar properties to cobalt, but its price is lower than cobalt. Its maximum energy density is 150A·h/kg, and its working voltage range is 2.5~4.1V; there is no overcharge. and overdischarge limitations, but its preparation requirements are extremely harsh. LiVO2 is less expensive and can form layered and spinel-type compounds. But when Li+ is deintercalated, its structure becomes unstable. LixMnyOz includes spinel-type LixMn2O4, orthorhombic LiMnO2 and layered LiMnO2, of which the most representative is spinel-type LiMn2O4, but it is easy to lose oxygen during heating and produce oxygen-deficient compounds with poor electrochemical performance. The preparation of high-capacity LiMn2O4 is more complicated.
2) Lithium intercalation organosulfides and their polymers
Such materials are new energy storage materials developed after the 1990s, including organic sulfides and their polymers, and are mainly used in lithium batteries with lithium as the negative electrode. The molecular structure of these materials contains disulfide bonds (—S—S—), and energy exchange occurs based on their reversible electropolymerization-electropolymerization process (2S-→S—S+2e-), and the theoretical energy density is as high as 1500~3500W·h·kg-1, the actual energy density can reach 830W·h·kg-1, and one of the great advantages of this material is that its organic groups and molecular structures can be controlled in a predetermined way, and its organic groups and molecular structures can be controlled in a predetermined way. mixed to change its physical, chemical and electrochemical properties.
2. Anode materials for lithium-ion batteries
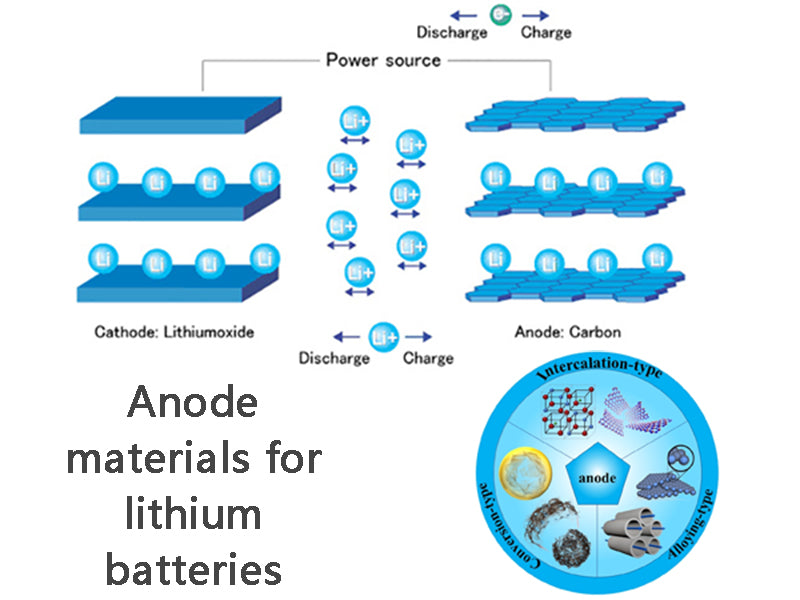
Compared with positive electrode materials, the innovation speed of negative electrode materials is faster. There are a wide variety of materials used for lithium-ion battery anodes. According to the basic composition of materials, they can usually be divided into two categories: carbon anode materials and non-carbon anode materials.
1) Carbon anode material
In lithium-ion batteries, reversible intercalation and delithiation carbon materials are used to replace the metal lithium anode in traditional lithium batteries, and the intercalation and deintercalation reactions of lithium ions in carbon negative electrodes are used to replace the deposition and dissolution reactions on pure lithium electrodes. The life cycle and safety of the battery are greatly improved. In addition to natural graphite, carbon materials for negative electrodes of lithium-ion batteries are usually prepared by heat-treating carbon-rich raw materials. Carbon materials for lithium-ion batteries can be divided into natural graphite, soft carbon and hard carbon. There are two kinds of natural graphite: flake graphite and earthy graphite (microcrystalline graphite). The carbon content of the former can be as high as 99% or more after beneficiation and purification. In the lithium-ion battery industry, flake graphite is often used as the raw material of carbon negative electrode. It has a good layered lithium storage structure, the theoretical lithium intercalation capacity can reach 372mA·h/g, and it is cheap and easy to obtain. It has the outstanding advantages of low discharge potential and stable discharge potential curve as a negative electrode material for lithium ion batteries. Soft carbon mainly includes petroleum coke, needle coke, carbon fiber, carbon microspheres, etc. It is a transitional carbon from amorphous carbon to graphite crystal, and is generally made from coal or petroleum as a precursor. Soft carbon is usually treated by high-temperature graphitization (2800°C or 3000°C) to be converted into artificial graphite and used as a negative electrode material for lithium-ion batteries. Hard carbon mainly includes resin carbon (such as phenolic resin, epoxy resin, etc.), organic polymer pyrolytic carbon (polyvinyl alcohol PVA, polyvinyl chloride PVC, polyvinylidene fluoride PVDF, polyacrylonitrile PAN, etc.) and carbon Black (such as ethyl fast black) is a carbon material close to amorphous structure. It is used as the negative electrode material of lithium battery. The reversible discharge capacity is generally between 500-700mA·h/g, and there is no voltage hysteresis phenomenon. The discharge capacity of hard carbon can be improved by adding impurity elements to the hard carbon material.
2) Non-carbon anode materials
Non-carbon anode materials can generally be divided into lithium-containing transition metal chlorides, transition metal oxides, and nano-alloy materials. Lithium transition metal hydride has good ionic conductivity, electronic conductivity and chemical stability, and is used as a negative electrode material for lithium ion batteries, and its discharge voltage is usually above 1.0V. However, it seems that further research is needed to achieve practical application of such materials. Transition metal oxides (tin-based oxides) such as SnO/SnO2 have the advantages of high specific capacity and relatively low discharge potential as negative electrodes for lithium-ion batteries. However, the first irreversible capacity loss is large, the capacity decays rapidly, and the discharge potential curve is not stable. By doping B, P, Al and other metal elements into tin oxides, amorphous composite oxides, ie amorphous tin-based composite oxides, are prepared, and their reversible capacity reaches more than 600 mA·h/g. The volume specific capacity is greater than 2200mA·h/cm3, and the cycle performance is also good.
3. Electrolytes for lithium-ion batteries
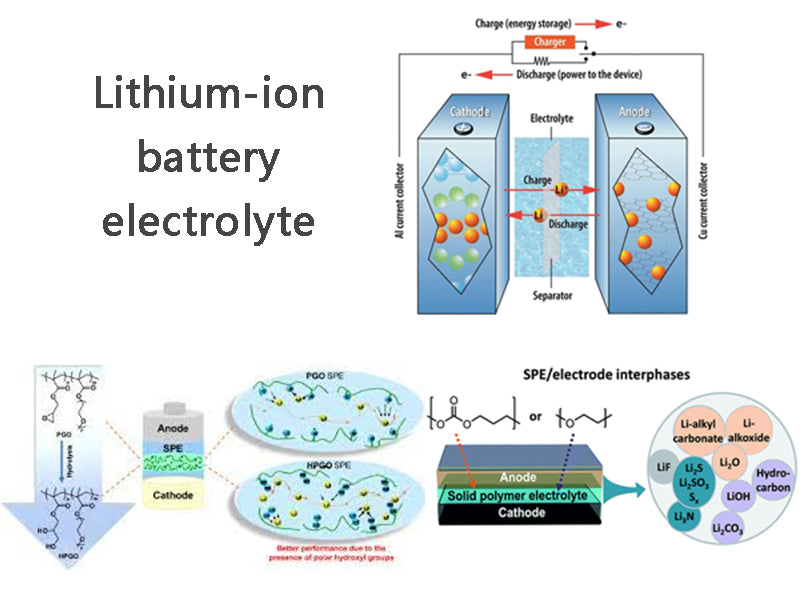
Lithium-ion batteries have many obvious advantages over traditional rechargeable batteries. In traditional batteries, the electrolyte is an electrolyte system with water as the solvent. Because water has good solubility for many substances and people have a deep understanding of the physical and chemical properties of aqueous systems, the choice of electrolytes for batteries is very wide. However, since the theoretical decomposition voltage of water is only 1.23V, even considering the overpotential of hydrogen or oxygen, the highest voltage of a battery with an electrolyte system using water as a solvent is only about 2V (such as lead-acid batteries). The voltage of lithium-ion battery is as high as 3~4V, and the traditional aqueous solution system does not meet its requirements, and a non-aqueous electrolyte system must be used as the electrolyte of lithium-ion battery. However, the conductivity of general solvents is limited and cannot meet the requirements of lithium batteries. Lithium salts with higher conductivity must be dissolved in the solvent to meet the requirements of lithium batteries. At present, LiPF6, LiAsF6, LiClO4, LiBF4 and LiCF3SO3 are mainly used as electrolyte salts in lithium ion batteries. When the above lithium salts are dissolved in a non-aqueous solvent, LiAsF6 and LiPF6 have the highest electrical conductivity; the second is LiClO4, the second is LiBF4, and the lowest is LiCF3SO3. Although LiAsF6 is easier to purify and does not decompose during disposal, its application is limited due to the toxicity of As in LiAsF6; LiClO4 may cause safety problems due to its strong oxidizing property; and LiPF6 is easily decomposed into PF5 and LiF, Therefore, LiBF4 and LiCF3SO3 were often used in the past. However, LiPF6 has the highest electrical conductivity, and through proper treatment, the electrolyte polymerization caused by its decomposition can be avoided. Therefore, LiPF6 is basically used as the electrolyte salt in current lithium-ion batteries. The ionic conductivities of various combinations of lithium salts and organic solvents are shown in Table 1.
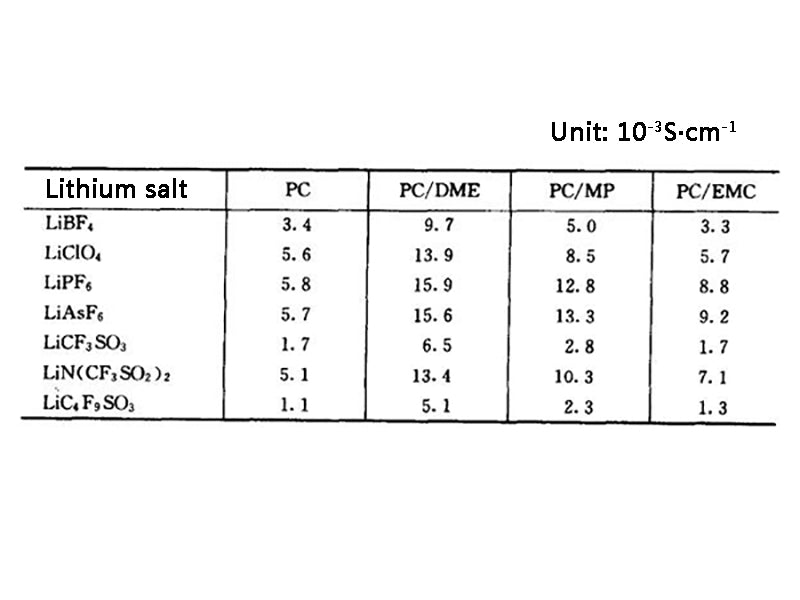
Table 1 Ionic conductivity of various lithium salts and organic solvent combinations
In the study of electrolytes, people are still looking for non-aqueous electrolytes with lower viscosity, larger dielectric constant and better stability; at the same time, they are also studying water-electrolyte systems and polymer solid electrolytes, which are carried out in the research of polymer solid electrolytes. research on composite materials. Broadly speaking, it can be divided into lithium-ion organic electrolyte batteries and lithium-ion polymer batteries according to different electrolytes. The former uses a liquid electrolyte, while the latter uses a solid electrolyte. At present, Japan's Sony, A&T Battery and Mat-sushita and other manufacturers represent the international advanced level of lithium-ion organic electrolyte batteries; and in terms of lithium-ion polymer batteries, it is Bellcore of the United States. Liquid electrolytes are mainly composed of lithium salts and mixed organic solvents, such as LiCIO4/PC (propylene carbonate) + DME (dimethyl glycol), PC + DME + EC (ethylene carbonate), EC + DEC (dicarbonate) ethyl ester), LiAsF6/EC+ THF (tetrahydrocarboxylate), etc. 1~2mol/L LiPF6/EC+DMC is currently recognized as the best electrolyte, but the preparation of LiPF6 is quite complicated and expensive.
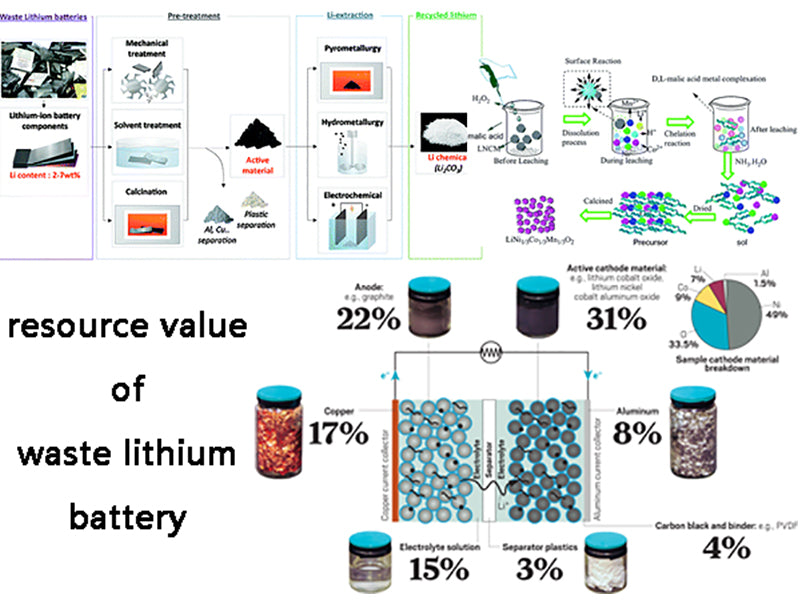
4. Recycling of lithium-ion batteries
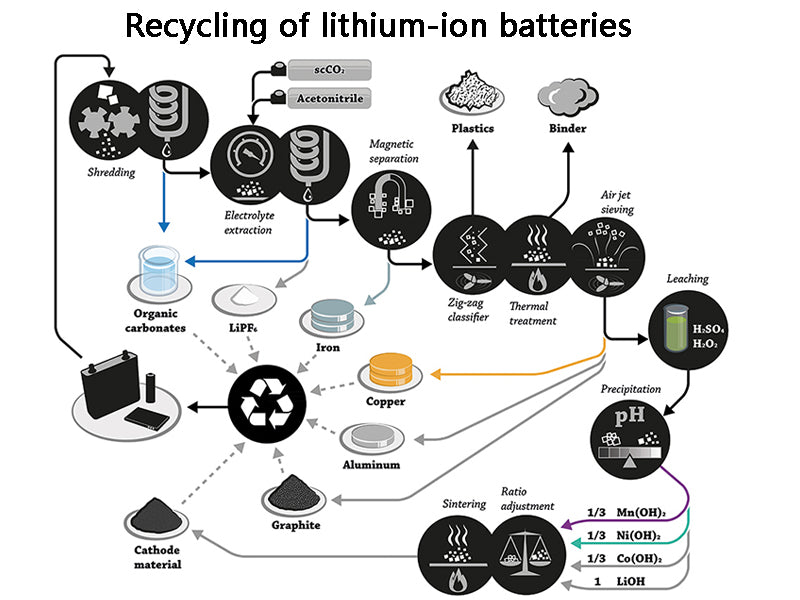
The so-called resource utilization refers to the technical method of taking measures to recover material and energy from solid waste, improving the circulation of material and energy, and creating economic value.
Taking a common mobile phone battery weighing about 40g as an example, it can be seen that the content of metal materials in lithium-ion batteries and the necessity of their reuse are shown in Table 2. The chemical compositions of several cobalt-containing concentrates are shown in Table 3.
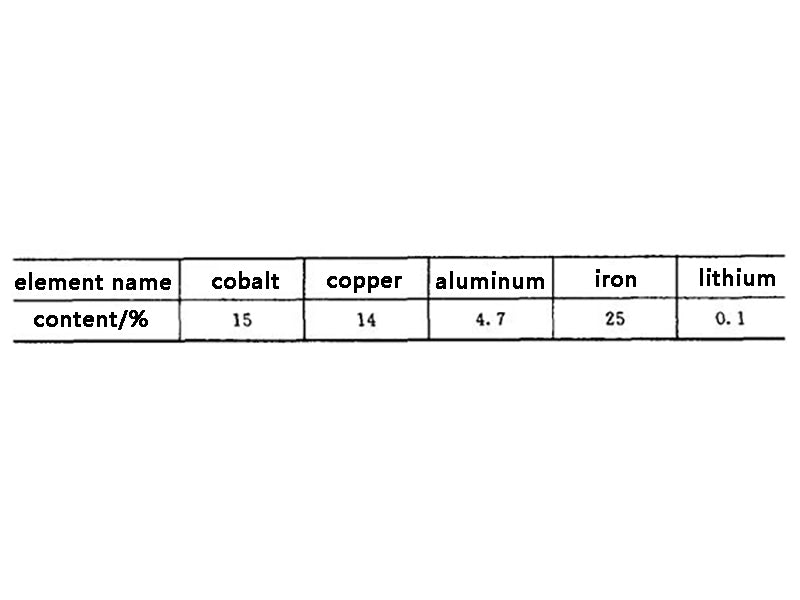
Table 2 Metal content in common lithium-ion batteries
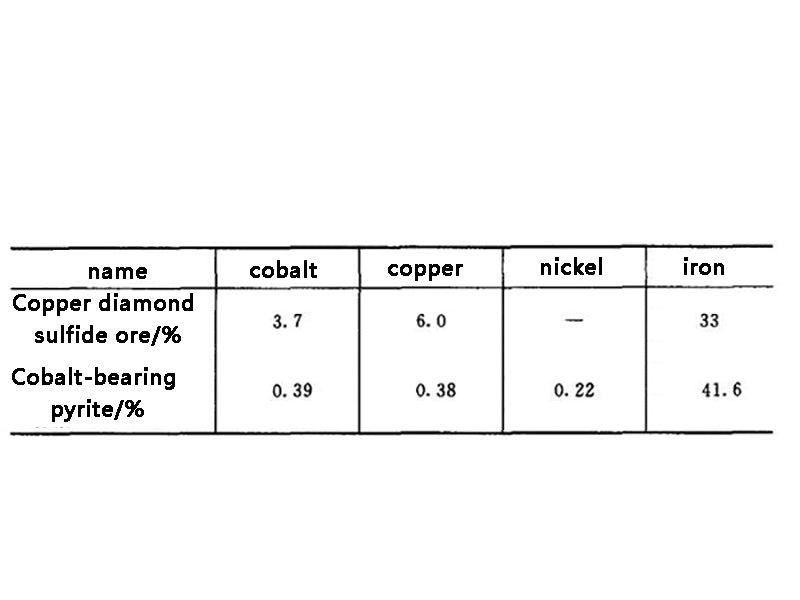
Table 3 Chemical composition of several diamond-bearing concentrates
Cobalt is a rare and expensive metal. It does not have a separate deposit, and is mostly associated with copper and nickel ores with low grades. All countries in the world pay more attention to the recovery of cobalt in industrial production. It can be seen from Table 1 and Table 2 that the waste lithium-ion battery is a kind of waste with high cobalt content. A battery weighing about 40g contains about 6g of metallic cobalt. If 100 million such batteries are scrapped each year, about 600t of recyclable cobalt is worth about 200 million yuan.