main content:
Lithium batteries include primary lithium batteries and secondary lithium batteries. Primary lithium batteries include Li/MnO2 batteries, Li/SOCl2 batteries and other primary lithium batteries. Secondary lithium batteries include lithium ion batteries, metal lithium negative electrode secondary batteries, aqueous lithium ion batteries, and the like.
Lithium has the smallest atomic weight (6.94), low density (0.534g/cm3, 20℃), smallest electrochemical equivalent [0.26g/(A·h)], and lowest standard electrode potential (-3.045V) among metals in the periodic table. Metal. Lithium-ion batteries appeared in the 1990s. It only took 2-3 years for lithium batteries to go from successful battery research to large-scale production.
1. Primary lithium battery
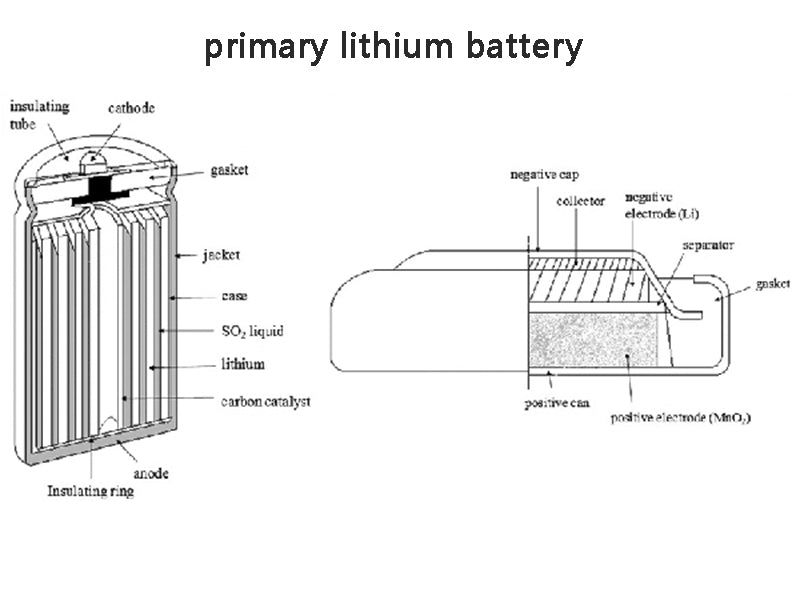
If an appropriate material can be selected as the positive electrode and matched with lithium, a higher electromotive force can be obtained. It is based on this consideration that the research and development of lithium batteries began worldwide in the early 1960s. Since metal lithium reacts violently with water, non-aqueous electrolytes were generally used as electrolyte solutions at that time, and CuF2 was mostly used as cathode material. However, these cathode materials are easily dissolved in electrolyte solutions. In addition, the initial battery structural materials cannot withstand long-term corrosion in the electrolyte solution, so there is no real commercial production of lithium batteries. After 1970, Japan's Matsushita Electric Company successfully developed a Li/(CFx)n battery, which solved the above-mentioned defects for the first time and was truly applied. In 1971, it was hailed as one of the top ten new products in Japan. In 1976, Japan's Sanyo Electric Company launched Li/MnO2 batteries, which have been widely used in calculators and other fields. From the earliest development of Li/(CFx)n batteries in the early 1970s, to the early 1980s, the production of lithium batteries in Japan increased significantly. The largest country in the promotion and application of lithium batteries.
At the same time, the United States established Power Conversion Co., Ltd. in 1970, specializing in the research of Li/SO2 batteries, and it was officially put into commercial production after 1971. The brand name was Eternacell, which was mainly used for military purposes. A promising lithium battery.
The French SAFT company began research on lithium batteries in the 1960s. The company's Dr. Gabano was the first to obtain a patent for Li/SOCl2 batteries in 1970. In 1973, American GTE Company and Israel Tadilang Industrial Co., Ltd. officially produced Li/SOCl2 batteries one after another. Israel Tadiran Industries Ltd., in cooperation with Tel Aviv University, built a factory in 1975. The plant was redesigned in 1977, built into mass production equipment and put into production, and began selling Li/SOCl2 batteries worldwide in 1978.
The lithium battery developed in the 1990s can provide a single cell voltage of up to 4V, and the energy density can reach 100~200W.h·kg-1, or even higher, and can be stored for a long time. Work within the temperature range (-40~70℃). At the same time, the demand for lithium batteries in the market has further expanded. Commonly used primary lithium batteries include Li/MnO2, Li/SO2, Li/SOCl2 and Li/CuO, etc., which have been widely used in electronic computers, cardiac pacemakers, beacons and Torpedoes, submarines, airplanes, cameras with automatic date marking, radios, electronic record books, and memory-supplementary power supplies, etc. Although the primary lithium battery has good performance, it is expensive and has the problems of poor safety performance, voltage hysteresis and high production conditions.
2. Secondary lithium batteries
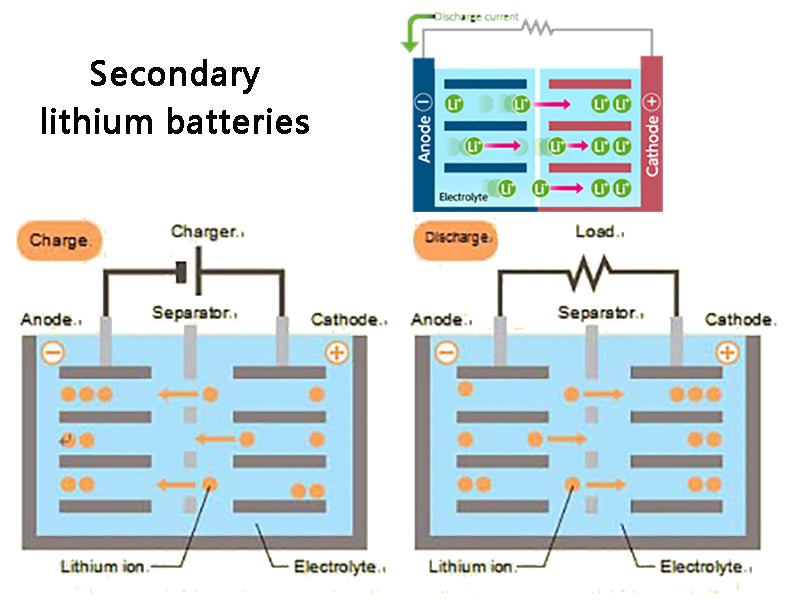
In 1964, he began to study the normal temperature secondary lithium battery. Lithium batteries using lithium as the negative electrode have the characteristics of high open circuit voltage (above 3V), high energy density (over 200W.h·kg-1 and 400W·h·L-1), stable discharge voltage, wide application range and long service life. . Lithium batteries are widely used in various fields, especially in cutting-edge technology and defense industry.
The higher the activity of the electrode material, the more irreversible the electrode reaction is. During the charging process of the electrode, metallic lithium will be deposited on the lithium negative electrode, resulting in dendrite-like crystals (dendrites). More seriously, the dendrites pass through the separator and connect the positive and negative electrodes, causing an internal short circuit in the battery, causing the battery to burn or even explode, resulting in serious safety problems. From a thermodynamic point of view, the high-purity lithium deposited on the lithium negative electrode during the charging process of the secondary lithium battery is very active, and part of the lithium will react with the electrolyte (or its impurities), making the charging and discharging efficiency of the secondary lithium battery very low. , thus affecting the life cycle of the battery.
There are two solutions to solve these shortcomings: one is to replace the liquid electrolyte with a polymer solid electrolyte, the so-called all-solid-state secondary lithium battery; the other is the rocking-chair rechargeable battery proposed by M. Armand. All-solid-state secondary lithium batteries have not fundamentally eliminated the problem of lithium dendrite formation. The rocking chair battery uses some materials with an open structure, which can allow lithium ions to be intercalated or deintercalated, and have a lower electrode potential to replace metal lithium as the negative electrode. For the positive electrode material, those materials with positive potential, small molecular weight, high energy density, A material with good electrochemical performance, good electrical conductivity, and a geometric void structure that allows intercalation and deintercalation of lithium ions. The active material of the electrode material is composed of lithium intercalation compounds, in which the low-potential material is used as the negative electrode active material, and the high-potential material is used as the positive electrode active material. The greater the potential difference between the positive and negative electrodes, the higher the electromotive force of the battery. When the battery is charged, lithium ions can be deintercalated from the positive electrode and inserted into the negative electrode through the electrolyte; when the battery is discharged, lithium ions can be deintercalated from the negative electrode and inserted into the positive electrode through the electrolyte. The operation process of the battery is actually the process of intercalation and deintercalation of lithium ions back and forth between the two electrodes, so this kind of battery is called "rocking chair battery", and its working principle can be seen in Figure 1.
In 1990, Japan's Sony Energy Development Co., Ltd. first replaced the original metal lithium with a lithium-intercalating negative electrode material, which overcomes the high activity of metal lithium and avoids the formation of lithium dendrites on the electrode surface. A working voltage of 3.6V has been successfully developed. , a new lithium-ion secondary battery with an energy density (mass) of 78W·h·kg-1, an energy density (volume) of 192W·h·L-1, a life cycle of 1200 times, and a monthly self-discharge rate of 12%, And quickly realize the commercialization of the battery. The working principle of this lithium-ion battery is exactly the same as that of the rocking chair battery proposed by M.Armand. Later, Canada Mori Energy Company successfully developed C/LiNiO2 lithium-ion battery. These batteries can not only maintain the advantages of primary lithium batteries, but also have excellent characteristics such as flexibility, light weight, long life cycle, excellent safety performance, high single-cell voltage and high energy density, and do not contain toxic heavy metals such as cadmium, lead, mercury, etc.; It has the characteristics of being environmentally friendly, low post-processing cost, and recyclable.
Lithium-ion secondary batteries generally include positive electrodes, negative electrodes, electrolytes, separators, positive and negative electrode leads, center terminals, insulating materials, safety valves, PTC positive temperature control terminals and battery shells and other accessories. Lithium-ion secondary batteries are mainly classified into two categories, lithium-ion batteries and lithium/polymer secondary batteries, according to different cathode materials. Lithium-ion battery refers to a secondary battery composed of two compounds that can reversibly intercalate and deintercalate lithium ions as positive and negative electrodes respectively; conductive polymers are used as positive electrode materials in lithium/polymer secondary batteries, such as polyacetylene, polymer Aniline etc.
The cathode materials of relatively mature lithium-ion batteries include lithium-cobalt oxide (LiCoO2), lithium-nickel oxide (LiNiO2) and lithium-manganese oxide (LiMnO4); the negative electrode has petroleum coke that can intercalate lithium ions (Li+). , graphite and layered graphite mixed carbon materials, etc. At present, the lithium-ion secondary battery market is mainly concentrated in mobile communications and notebook computers, accounting for more than 90% of the entire lithium-ion battery. For example, in 2002, the total output of lithium-ion batteries was 862 million, and more than 500 million were used for mobile In terms of communication, more than 200 million are only used for notebook computers, and more than 100 million are only used for video cameras and digital cameras.
In 1994, Bellcore Corporation first pointed out the possibility of using a class of materials containing polymers and conducting electricity as electrolytes to manufacture rechargeable lithium batteries, namely polymer lithium ion secondary batteries. The positive electrode of the polymer lithium secondary battery uses cobalt oxide lithium, the negative electrode uses a graphitized carbon material, and aluminum and copper are used as current collectors. The shape, area, quality, and safety of such batteries have been greatly improved, and they are quickly favored by major battery companies. However, the proportion of polymer lithium ion secondary batteries in the entire lithium ion secondary battery is still small, accounting for about 6% to 7% of lithium ion secondary batteries in 2002, and its market is mainly concentrated in personal digital devices. About 41%; applications on mobile phones account for 8%; and applications in notebook computers only account for about 2%.
The all-solid-state metal secondary lithium battery is still in the development stage. Hygro-Quebec of Canada and 3M Corporation of the United States have carried out fruitful research and development work under the sponsorship of the USABC. The all-solid-state metal secondary lithium battery uses a cross-linked PEO-based copolymer and uses metal lithium as the negative electrode. Its theoretical capacity is more than 10 times that of the carbon negative electrode, and the safety of the battery is also substantially improved.
3. Application of lithium battery
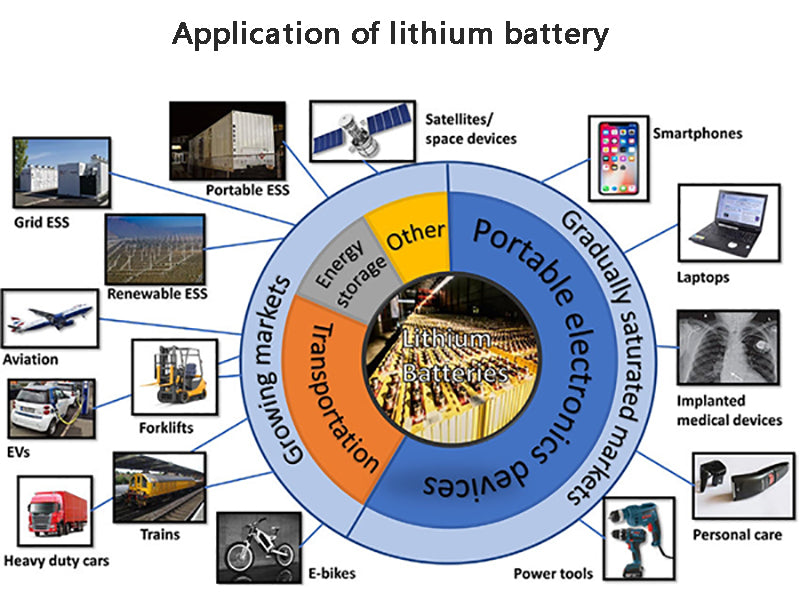
Because lithium batteries have many advantages such as high specific energy, flat discharge voltage, wide operating temperature range, long wet shelf life, etc., and do not contain mercury, cadmium, lead and other metals that are harmful to the environment, they have been used in military and daily life. have been widely used. The applications of primary lithium batteries and secondary lithium batteries can be roughly divided into three types: general consumption, industrial and medical applications, and military applications. Consumer applications can be roughly divided into three categories, namely household goods, portable products and automotive supplies. Household products are most commonly used as power sources for record players, telephones, alarm clocks, watches, cameras, car radios, etc. Industrial and medical applications can be divided into safety, high temperature testing, measurement and other aspects, mainly used in anti-theft equipment, circuit control in large department stores and factories, typewriters, oil well drilling equipment, pacemakers, etc. Military use is mainly used as a memory backup battery, power supply and so on.
The United States and Japan are the world's largest producers of lithium batteries. Lithium batteries in the United States are focused on military use. Japan focuses on civilian use and almost monopolizes the world's civilian lithium battery market.
4. The development trend of lithium-ion batteries
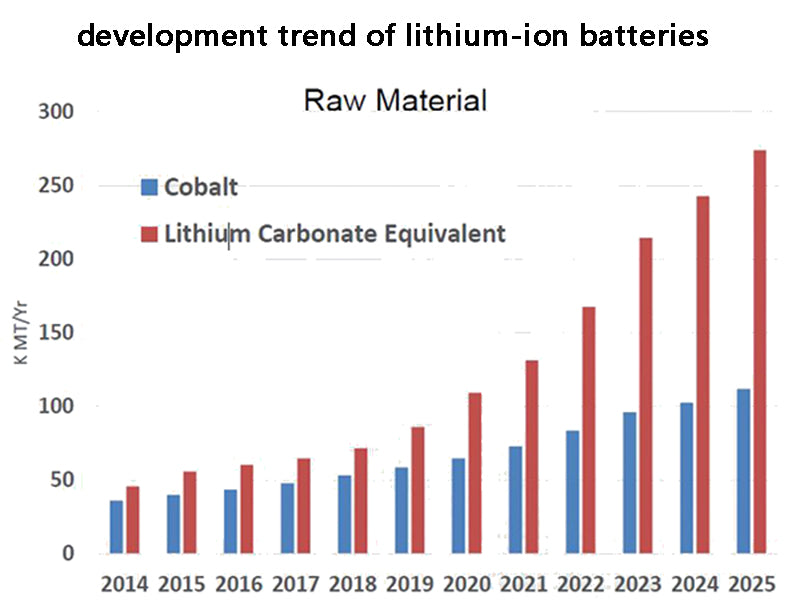
The development trend of lithium-ion batteries is as follows: ① from liquid lithium-ion batteries to solid-state (polymer gel electrolyte) lithium-ion batteries; ② in the three existing lithium cobalt oxides, lithium nickel oxides and lithium manganese oxides In the lithium-ion battery of 2000, lithium manganese oxide is a research hotspot, and the key problem is to solve the poor cycle performance and high temperature capacity fading; lithium nickel oxide is also the focus of attention, by doping cobalt or other elements, it can be produced with good capacity and cycle performance The prospects of both are promising; ③ The research and development of non-carbon anode materials, metal lithium or lithium alloys as anode materials is also very promising; ④ Due to the need for mobile phones to develop in a small and lightweight direction, square lithium-ion batteries will replace Cylin.