Certain oxo acid lithium salts such as Li3PO4 and Li4SiO4 have high Li+ conductivity at high temperatures. The conductivity of some oxyacid double salts, especially those series compounds with γⅡ-Li3PO4 type structure, is higher than that of LiI-Al2O3 at low temperature or even at room temperature. The γⅡ-Li3PO4 type structure belongs to the orthogonal rhombohedral system, in which oxygen ions are densely packed in the hcp lattice, and the octahedral gap formed by it is only partially occupied by cations, and Li+ can be conducted in the a-b plane through the empty gap position. But the conductivity of the compound in the ideal γⅡ structure is usually very low, because all the lithium ions are used to build the framework of the structure. Therefore, in order to obtain a high-conductivity solid electrolyte, the ratio of cations in the oxo acid salt such as Li4SiO4 [(Li+Si): oxide ion] cannot be less than 1.
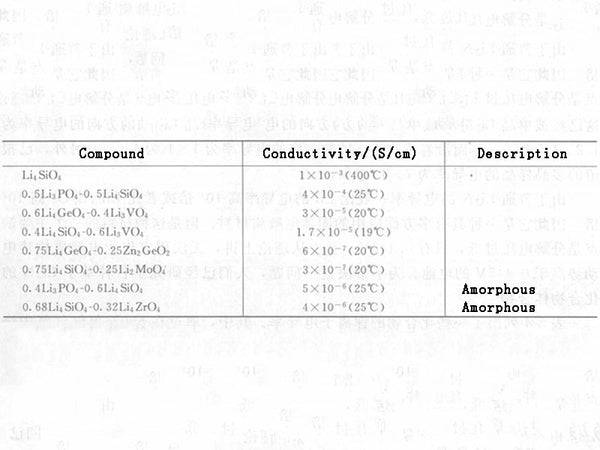
The compound xLi3PO4-(1-x)Li4SiO4 can be rewritten as a defective γⅡ form: Li*1-xLi3Si1-xPxO4, where Li* represents conductive lithium ions in the a-b plane, that is, these lithium ions do not participate in the construction of the structural framework of the material. The conductivity of this solid electrolyte reaches its maximum value (4×10-6S/cm) when the x value reaches 0.5, which means that half of the cation sites in the a-b plane are occupied. Some complex oxo acid salts that do not contain PO4 groups, such as xLi4GeO4-(1-x)Zn2GeO4 (when x=3/4, become LISICON) or xLi4GeO4-(1-x)Li3VO4 also belong to the γⅡ structure. As shown in the table in Figure 1, it exhibits very good lithium ion conductivity.
These compounds (in the Li4SiO4-Li3VO4 system) are synthesized as follows: toluene is used as a dispersant, and an appropriate amount of high-purity Li2CO3, SiO2 and V2O5 are thoroughly mixed. After evaporating the toluene, the mixture was heated at 600°C to remove CO2, and then sintered at 700°C for 40h, and the product obtained was ground into fine powder. Then, it is pressed into a cake under a pressure of several tons per square centimeter, and sintered at 1000°C for 1 hour to obtain the final solid electrolyte. The solid electrolyte of other components can be prepared in the same way.
The amorphous film of xLi3PO4-(1-x)Li4SiO4 is made on the substrate by sputtering technology in an Ar-40% oxygen atmosphere (total pressure 3Pa) using excess lithium as a target. At 25°C, the conductivity of the Li3.6Si0.6P0.4O4 film prepared in this way can reach 5×10-6S/cm, which is greater than the conductivity of the crystalline phase bulk material of the same composition. Due to the amorphous nature of this film, the details of the structure are unclear. However, these films may have a γⅡ-Li3PO4 type structure, in which the arrangement of SiO4 and PO4 tetrahedrons is somewhat disordered. The higher conductivity observed in the film may be due to the wider window in the conductive channel caused by the disordered arrangement.
The electrical conductivity properties of solid electrolytes based on oxo acid salts are summarized in Figure 1. It should be pointed out that unlike Li3N or LiI-Al2O3 systems, these solid electrolytes are very stable in general environments.
The perovskite structure material ABO3 (where A=La or Li; B=Ti) has a high lithium ion conductivity, with a bulk conductivity of 1.2×10-3S/cm at 30°C, and an apparent grain boundary conductivity of 0.03×10-3S/cm. The stable voltage of this solid electrolyte is only 1.6V (compared to metallic lithium), below which Ti4+ will be reduced to Ti3+. There is a perovskite structure Li-Sr-Ta-Zr-O with higher conductivity, in which the A and B cations in SrZrO3 are replaced by Li and Ta, respectively. The lithium ion conductivity of the optimized composition Li3/8Sr7/16Ta3/4Zr1/4O3 at 300℃ is 0.2×10-3S/cm, and the apparent grain boundary conductivity is 0.13×10-3S/cm. This electrolyte is stable above 1.0V (relative to metallic lithium). Below 1.0V, about 0.08 mol of lithium can be irreversibly inserted into 1 mol of Li3/8Sr7/16Ta3/4Zr3/4O3.