Nano oxide

According to the lithium storage mechanism, there are two main types of nano-oxides: alloy lithium storage mechanism and redox reaction mechanism. The former is mainly tin oxide, and the latter includes nano-oxides of Co, Ni, Cu and Fe.
① Nano tin oxide
To improve the high-current performance of tin oxide, the preferred method is to synthesize nanoparticles. The synthesis methods of nano tin oxide include chemical vapor deposition method, gel-gel method, template method and so on. Taking the template method as an example, the template method as shown in Figure 1 can be used to synthesize nano-sized SnO2. The process is as follows: using a nanoporous polymer as a template, immersing it in a tin-containing solution, and then attaching it to a current collector, after removing the solvent, the polymer is removed by plasma to obtain SnO2 nanofibers; when heated in the air, it becomes crystalline SnO2 nanofiber particles, and its dispersion is single, 110nm, just like a comb, its fast charge-discharge performance is good, the capacity is more than 700mA·h/g when charging and discharging at 8℃, and the capacity decays very slowly. The reason is that nanoparticles slow down changes in volume, thereby improving cycle performance. Both the capacity, the charge-discharge performance under high current, and the cycle performance are better than the behavior of SnO2 film. In addition, the use of microwave-elevated solution methods and the use of surfactants as templates can synthesize tin nano-oxides.
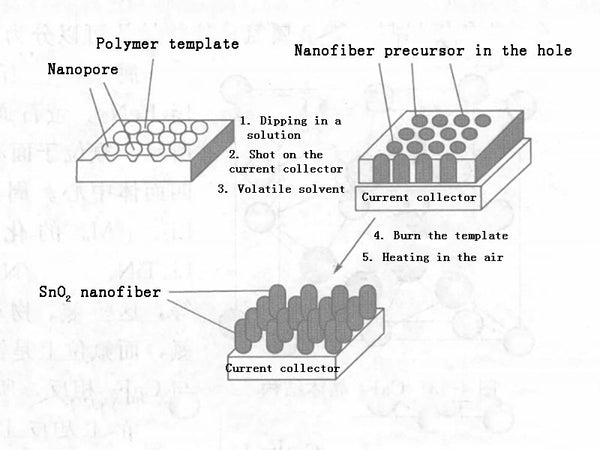
Figure 1-Schematic diagram of the process of preparing nano-SnO2 by template method
② Other nano metal oxides
As mentioned earlier, nano-transition metal oxide MO (M=Co, Ni, Cu or Fe) has excellent electrochemical performance, its reversible capacity is between 600~800mA·h/g, and the capacity retention rate is high. It can be 100% after 100 cycles, and has fast charge-discharge capability; the voltage plateau when lithium is inserted is about 0.8V, and when lithium is released, it is about 1.5V.
Micron-sized Cu2O, CuO, etc. can also reversibly store lithium, and the capacity is relatively high. The mechanism is similar to the above-mentioned nano-sized CuO and other oxides. The above-mentioned micron or nano oxides can also be doped. For MgO doping, the lithium storage mechanism is consistent with that of undoped oxides. Although the initial capacity decreases after doping, the capacity retention rate or cycle performance is improved. However, the cycle performance and reversible capacity of inorganic oxide negative electrode materials are not only affected by the particle size, but the crystallinity and particle morphology also have a great influence on the performance. Through optimization, the comprehensive electrochemical performance of oxide anode materials can be improved.
Nano metals and nano alloys
Nano metal mainly refers to nano tin. The lithium storage performance of silicon is similar to that of tin, and both can form reversible compounds up to Li22M4, and the theoretical capacity is up to 4000mA·h/g.
① Nano tin and its nano alloy
The reversible capacity of tin is very high, but the volume changes greatly during the charging and discharging process, as high as 600%. Therefore, during the reversible insertion and de-intercalation of lithium, its microstructure is destroyed and tin is powdered, resulting in a rapid decrease in capacity. One of the effective methods currently used is to prepare nanomaterials. Because the specific surface area of the nanoparticles is large, it is beneficial to buffer the volume changes during the charging and discharging process. Of course, the specific surface area effect of nanoparticles is also conducive to the deintercalation of more lithium.
The liquid crystal template method is mainly used to make tin into a nano-structured electrode film with a particle size of 3-10nm. Since the expansion and contraction caused by the insertion and extraction of lithium will not damage the structure of the nano-tin, not only the capacity is high, but also the cycle performance is better than that prepared by the non-template method.
Doping with nanoparticles can further improve the cycle performance of the electrode. For example, when the SnSb particle is smaller than 300nm, it can reach 360mA·h/g after 200 cycles. When the SnSb alloy size is at the nanometer level, the cycle performance is significantly improved, and the capacity is as high as 550mA·h/g.
② Nano silicon and its nanocomposite
The performance of silicon is similar to that of tin, and its reversible capacity is high, but its cycle performance is not ideal. The main method of improvement is to make it into nanoparticles. During the reversible insertion and deintercalation of lithium, nano-silicon is transformed from amorphous to crystalline silicon, and nano-silicon particles will agglomerate, causing the capacity to decay as the cycle progresses. The cycle performance of the amorphous nano-silicon film prepared by chemical vapor deposition method is not good. The submicron film (500nm) of amorphous silicon is prepared, and its reversible capacity can be as high as 4000mA·h/g. Through the control of the termination voltage, the cycle performance can be improved, but its reversible capacity is reduced. In order to improve the electrochemical performance of nano-silicon, doping treatment is generally performed. For example, dispersing silicon into an inactive TiN matrix to form a nanocomposite material, although the capacity is low (about 300mA·h/g), but the cycle performance is very good, and the preparation method is very simple, and only high-energy mechanical grinding is required. This is due to the fact that silicon is uniformly dispersed in the nano-TiN matrix, and the volume of the electrode changes continuously when lithium is inserted and extracted, rather than abruptly. The longer the milling time, the better the silicon dispersion and the better the cycle performance. When the silicon is uniformly dispersed in the silver carrier, because the silver carrier has high conductivity and flexibility, and the silicon is in the form of nanoparticles, the volume change of silicon during charging and discharging is greatly buffered, and the cycle performance is ideal.