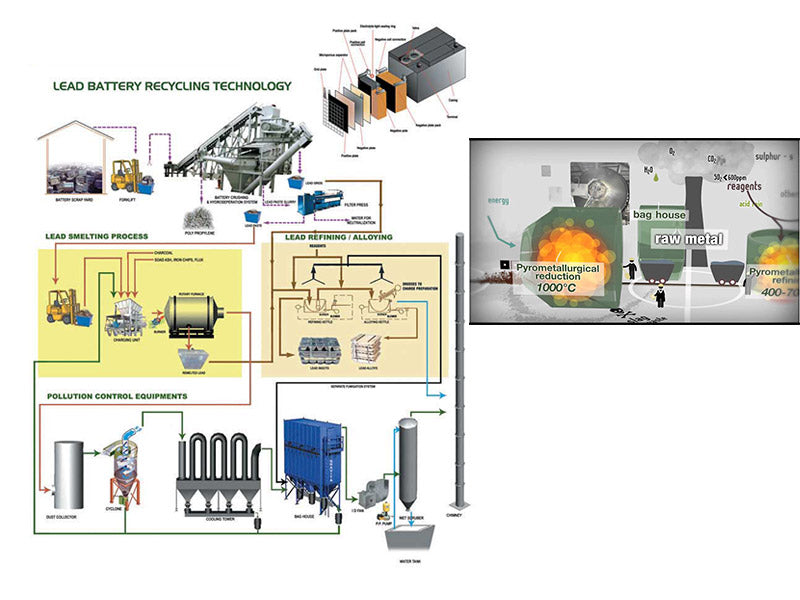
main content:
Battery debris from lead-acid battery disassembly and pretreatment processes is actually a mixture of metallic lead, lead oxides, lead sulfates, and other metals such as calcium, copper, silver, antimony, arsenic, and tin. In order to separate the metal lead from the mixture, two methods can be taken: high temperature smelting technology (or smelting technology), hydrometallurgy technology (or electrolysis).
The reduction effect of PbO2 has an important influence on the whole recovery technology, and its reduction process mainly includes pyrometallurgy and hydrometallurgy.
①Pyrometallurgy is to reduce and smelt PbO2 and other components PbSO4, PbO, etc. in the slag into Pb in a metallurgical furnace. However, due to the production of secondary pollutants such as SO2 and high-temperature lead dust, and high energy consumption and low utilization rate, it will be gradually eliminated.
②Hydrometallurgy is to add reducing agent to reduce PbO2 into low-valence lead compound under solution conditions. A number of reducing agents have been tried. Among them, the reduction of PbO2 by FeSO4 in sulfuric acid solution is ideal and has industrial application value. PbO2 is reduced by FeSO4 in sulfuric acid solution, and the reduction process can be represented by the following formula.
PbO2(s)+2FeSO4(l)+2H2SO4(l)→PbSO4(s)+Fe2(SO4)3(l)+2H2O
This reduction process is stable and fast, and can also completely convert the metallic lead in the sludge, which is beneficial to the reduction of PbO2.
The reducing agent in the lead reduction process can be prepared by using the iron and steel pickling wastewater to achieve the purpose of treating waste with waste. Pyrometallurgy is to reduce all metal compounds to metals or corresponding reduction products by heating and adding enough fluxes and reducing substances. Usually added reducing agents are coke, coal powder and so on. The environmental impact of this process mainly comes from the release of lead compounds, scum, lead-containing dust filters, sulfur dioxide, tar, chlorine, and chlorides from the combustion of organic matter during battery dismantling.
The purpose of electrolysis in hydrometallurgy is to selectively reduce all lead compounds into metallic lead by means of electricity, and the main pollutants are wastewater containing organic and inorganic substances.
1. High temperature smelting technology

The purpose of this technology is to chemically reduce the metal compound content by thermal calcination and reduction. Before melting, the sulfur in lead sulfate can be removed by reacting with a mixture of sodium carbonate and sodium hydroxide, and sometimes it can also be achieved by reacting ferric oxide and calcium carbonate. This process reduces the amount of slag by means of smelting, and also reduces sulfur dioxide emissions. However, in addition to desulfurizers, there are other ways to control sulfur in lead sulfate.
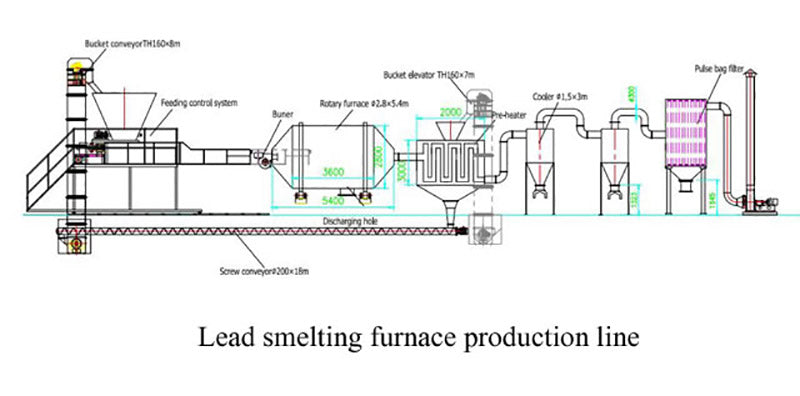
The acidic electrolyte must be treated before being sent to the melting furnace: neutralized with sodium hydroxide, so that all the lead is precipitated in the form of lead hydroxide. These compounds are then filtered and poured directly into the furnace. The remaining sodium sulfate solution can be further purified to obtain high purity salts.
The metals from the desulfurization and neutralization steps are fed into the furnace together with lead compounds and smelting effluent and reducing medium. The required energy sources are oil, natural gas, coal, electricity, etc., depending on the specific method and requirements. There are also some different devices in the smelting process such as rotary furnace, reverberatory furnace, blast furnace, electric furnace, rotary kiln, etc.
The melting temperature of the smelting medium is lower than the melting temperature of lead, and its addition is not only to reduce the melting temperature of lead, but also to provide a liquid solvent that can remove some unwanted compounds during the smelting process. The smelting medium is contaminated with various impurities, thus forming slag. The physical and chemical properties of the slag play an important role in the subsequent processing, and these properties depend largely on the composition of the smelting medium used.
The purpose of adding a reducing medium is to extract metallic lead from lead oxides and lead hydroxides, and carbonaceous materials such as coke are usually used.
The number of additions of smelting medium and reducing medium must be well controlled. Insufficient smelting medium will not remove all sulfur and other impurities from the battery fragments, and will discharge a larger amount of sulfur oxides. On the other hand, if the amount of reducing medium added is insufficient, it will not be able to reduce all lead oxides, and the lead content in the slag will be high, which will also become an environmental hazard.
In the final process, molten lead metal will settle on the bottom of the furnace. However, these lead sometimes contain a lot of metal impurities with economic value, so when it is necessary to recover the lead with higher purity, it must be refined.
2. Hydrometallurgy technology
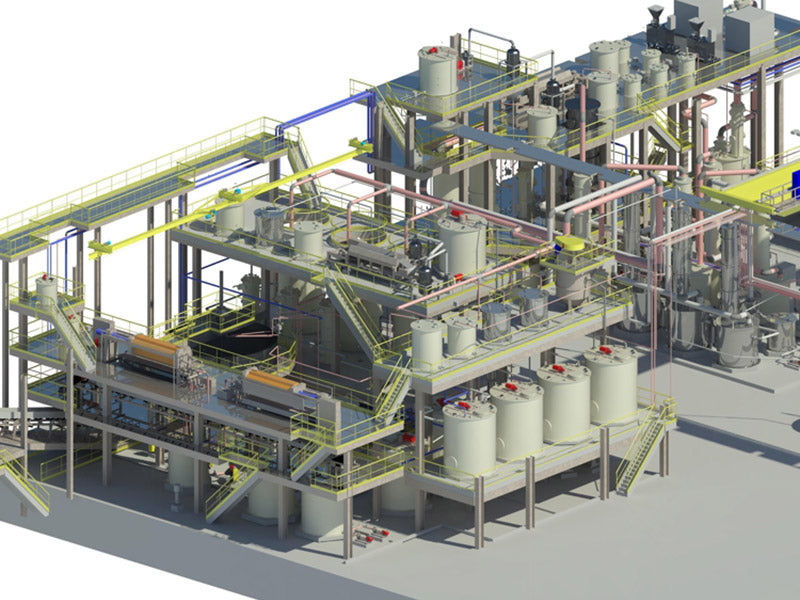
The purpose of hydrometallurgy, or electrolysis, is to convert the lead in all lead compounds into metallic lead. Although this method is more expensive for a separate refinery, it provides better separation. When the raw material is properly pretreated, it will be an effective technical means for refining lead, and can achieve good results. The chemical principle of this method is to first convert the lead in all compounds into divalent lead, and then convert the divalent lead into metallic lead through electrolysis. The crystalline or spongy electrolytic deposits of lead are continuously dropped from the electrodes to the conveyor belt to be collected, pressed into a high-purity lead cake, and then sent to the furnace to be cast into ingots. The whole refining process can run continuously for 24h without interruption.