
Main content:
The aluminum air battery uses light metal aluminum as the anode active material and oxygen in the air as the cathode active material. It has the advantages of large capacity, high specific energy, low cost, and no pollution, and is considered to be a battery with great development potential and application prospects in the future. Besides, liquid metal battery is also widely used in many fields.
The research work of aluminum air battery can be divided into aluminum anode, air electrode and battery structure. The theoretical specific energy of the aluminum-air fuel cell can reach 8100Wh/kg. As a special fuel cell, aluminum air battery has great commercial potential in military, civil, and underwater power systems, backup power sources for telecommunication systems, and portable power supplies.
Features of aluminum air battery
- High specific energy. Aluminum air battery is a new type of high specific energy battery. The theoretical specific energy can reach 8100Wh/kg. The products currently developed can reach 300-400 Wh/kg, which is much higher than the specific energy of various types of batteries today.
- The specific power is medium. Because the working potential of the air electrode is far away from its thermodynamic equilibrium potential, the exchange current density of the aluminum air battery is very small, and the polarization of the battery is very large when the battery is discharged, so that the specific power of the battery can only reach 50-200 W/kg.
- Long service life. The aluminum electrode of the aluminum air battery can be replaced continuously, so the lifespan of the aluminum air battery depends on the working life of the air electrode.
- Non-toxic and no harmful gas is produced. The electrochemical reaction of aluminum air battery consumes aluminum, oxygen and water to generate Al2O3·nH2O, which can be used for drying adsorbents and catalyst carriers, grinding and polishing abrasives, ceramics and excellent precipitants for sewage treatment, etc.
- Strong adaptability. The structure of aluminum air battery and the raw materials used can be changed according to the practical environment and requirements, and it has strong adaptability.
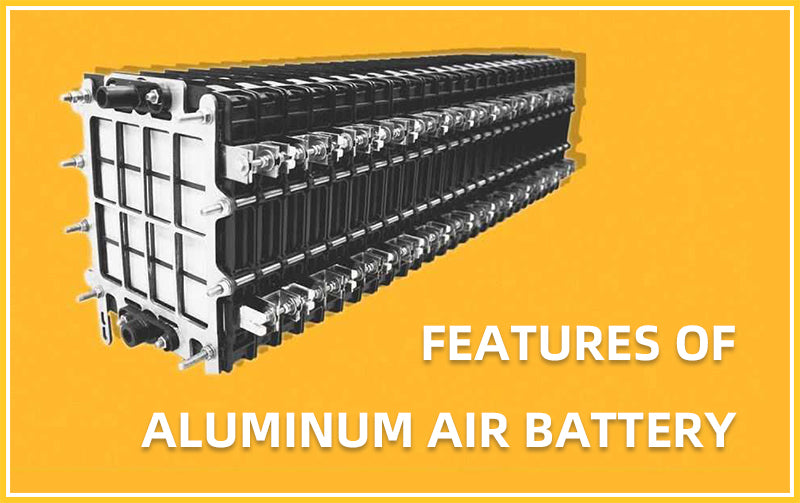
Aluminum, the raw material of battery negative electrode, is cheap and easy to obtain. Compared with other metals, the price of metal aluminum is relatively low, and the manufacturing process of metal anodes is relatively simple.
Composition of aluminum air battery
The electrolyte of aluminum air battery is mostly neutral salt solution or strong alkaline solution. When a neutral electrolyte is used, the self-corrosion of the anode is small, but the surface passivation of the aluminum anode is serious, which reduces the working voltage. At this time, it is difficult to increase the power and current of the battery, and it will also lead to voltage lag, and the product aluminum hydroxide colloid will also settle and block the electrolyte, so this type of battery can only be used as a low-power power output device.
When a strong alkaline electrolyte is used, the passivation of aluminum is reduced, and the lye can absorb a certain amount of reaction product aluminum hydroxide, and the performance of the battery is relatively good, but aluminum is an amphoteric metal, which will occur in a strong alkaline environment. Strong hydrogen evolution corrosion, releasing a large amount of hydrogen, reduces the output power and anode utilization of the battery, and is more serious at high current density.
The cathode of the aluminum air battery is the reaction place of O2, which has the functions of air permeability, conductivity, waterproof, corrosion resistance and catalysis, and is also often called the air electrode. The air electrode is generally composed of a three-layer structure of a porous catalytic layer, a conductive current collector and a waterproof and breathable layer: the porous catalytic layer is the main place where oxygen is reduced. The junction of the diffused oxygen, oxygen reduction catalyst and thin-layer electrolyte forms a three-phase interface electrochemically active site.
The conductive current collector mainly plays the role of electrical conduction and mechanical support. the waterproof and breathable layer has a loose porous hydrophobic structure, which is both a catalytic layer provides the gas required for the reaction and prevents the electrolyte from flooding the gas diffusion channels. The catalytic layer is the most critical part of the air electrode and plays a decisive role in its electrochemical performance.
The performance of the aluminum air battery depends largely on the selected cathode catalyst. The performance of the air electrode can directly affect the electrode reaction balance. Therefore, improving its performance can improve the utilization rate of the anode of the aluminum air battery to a certain extent and inhibit the self-corrosion of the anode aluminum.
Factors influencing the development of aluminum anodes in aluminum air battery
There is a passivation film on the surface of aluminum, which affects the electrochemical activity of aluminum. Aluminum is an amphoteric metal element, which determines that it is prone to hydrogen evolution and corrosion in a strong alkaline environment, which affects the electrode potential, and the product floats in the electrolyte to affect the entire electrochemical reaction.
The unique semi-open system of the aluminum air battery makes the air electrode vulnerable to the influence of external humidity, and the aluminum anode "submerges" or "dries up", and even "climbs" or "leaks". This will cause damage to the structure of the entire air battery. In order to solve the above problems, experts propose the following methods:
Aluminum anode alloying
Industrial grade aluminum (99.0%) contains many impurities, such as iron (0.5%), silicon, copper, manganese, magnesium and zinc, which will aggravate the hydrogen evolution corrosion of aluminum at the phase interface, especially iron and aluminum will form localized galvanic cells, resulting in exponentially increased electrochemical corrosion. Alloy components that can improve both chemical activity and corrosion resistance can be added to aluminum.Add slow release agent to electrolyte
Due to the cost of anode alloying, it is often chosen to add some slow-release agents to the electrolyte to ensure the performance of the aluminum air battery. Some carboxylic acid, amine and amino acid slow-release agents and their inhibitory efficiency on aluminum corrosion. Using natural substances as inhibitors of metal aluminum corrosion, experiments have shown that organic amines, pyrroles, etc. have obvious inhibitory effects on aluminum corrosion.
By adding organic compounds and water-soluble compounds to strong alkaline electrolytes, the electrochemical behavior of aluminum metal anodes was studied to reduce the corrosion rate of aluminum, thereby improving the performance of aluminum-air batteries.
Heat treatment process
Heat treatment affects the properties of the alloy by changing the distribution of trace elements in the aluminum alloy and the microstructure of the alloy surface, which belongs to the research category of technology. The best heat treatment process can be found by suitable orthogonal experiments.

Commonly used catalysts for aluminum air battery
- Precious metal catalysts. Platinum and silver are commonly used, and their catalytic activity and high performance are relatively stable, but their adoption rate is not high due to their high price and shortage of resources.
- Metal macrocyclic compound catalyst. Organometallic macrocycles have good catalytic activity for oxygen reduction, especially when they are adsorbed on large surface area carbons. And their activity and stability can be significantly improved by heat treatment. Therefore, it is expected to replace noble metal oxygen reduction catalysts. Common synthesis methods of metal macrocyclic compounds include thermal decomposition method and precursor preparation method.
- Perovskite oxide catalysts. Perovskite oxides have high catalytic activity for the reduction and evolution of oxygen and are inexpensive, so they have broad application prospects in aluminum-air batteries and fuel cells. The current research on perovskite oxygen electrode catalysts mainly focuses on improving the preparation method and finding new substitution elements to improve the catalytic performance.
- Inexpensive catalysts. The most important representative is manganese dioxide catalyst. Its biggest advantage is that it is rich in raw materials and low cost. It can be widely used in batteries with aqueous or non-aqueous electrolytes. However, the electrocatalytic activity of a single manganese dioxide is limited. Research has never stopped.
- AB2O4 spinel oxide catalyst. The crystal lattice of spinel is face-centered cubic. There are 32 close-packed O2- ions in the unit cell, and 64 tetrahedral voids and 32 octahedral voids are occupied by metal ions. The dehydration activity of spinel is related to the fraction of B ions located in the tetrahedral voids.
- Other metal and alloy catalysts. Alloy catalysts such as nickel-iron and nickel-cobalt are also often used, which have good catalytic activity and corrosion resistance, and are also a feasible catalyst direction for aluminum-air batteries.
- Composite catalyst. Combining two or more catalysts together can better improve the catalytic activity of the air electrode of the aluminum air battery.
Application prospect of aluminum air battery
At present, aluminum-air batteries have not been widely used in industrial and civil fields, mainly due to the need to improve the material preparation technology and the understanding of the concept of secondary charge and discharge. At the technical level: the measured specific energy and discharge efficiency of the aluminum air battery are quite different from the theoretical values.

The main technical problems include:
- The self-corrosion and hydrogen evolution of the aluminum anode largely restrict its discharge efficiency, and the surface passivation of the aluminum anode affects its discharge response aging.
- The matching between the electrolyte and the anode can not only form a fast anodic oxidation reaction response mechanism with the aluminum electrode, but also maintain the high efficiency and stability of ion transfer and the recyclability of oxidation products.
- The structure of the air electrode, the self-loss of the current in the conductive current collector, and the oxygen reduction capacity of the air electrode catalyst need to be further optimized and improved.
The concept of aluminum air battery as a secondary battery: As a metal fuel cell, the general concept of aluminum air battery is that it is a primary battery, which is a misunderstanding in the understanding of the charge-discharge cycle unit. As a classic secondary battery, the lithium-ion battery that we generally use now can realize instant charge-discharge conversion.
If the aluminum air battery can realize the industrialized charge-discharge process, it can also be regarded as a secondary battery from the perspective of this large cycle system. secondary battery, and this is one of the key technologies to solve its popularization and application. The high specific energy of aluminum air battery and its safety and harmless to the environment determine that it will have a broad development prospect.
In the future, it may be first used in mobile equipment such as power vehicles and mining. Mining companies have made great progress in the upstream of the battery industry chain now. Here is an article of top 5 lithium mining companies in China for you to get more relevant information. The aluminum air battery can be designed as an integrated battery pack, which is stored at a gas station or a special charging station like gasoline and other fuels.
When the anode is used up during use, the battery pack can be directly replaced. The discharged battery pack is separated and recovered by a professional technical company and the secondary assembly of the battery pack is carried out.
Related article: metal air battery, top 10 lithium ion battery anode material companies in China, photovoltaic industry