The characteristic of this type of polymer electrolyte is that the polymer electrolyte only contains two basic components: polymer (mostly using PEO) and alkali metal salt LiX. It is the most studied polymer electrolyte system so far. The main chain of this type of polymer contains a strong electron-donating group-ether oxygen functional group, so PEO is one of the main complexes with better complexing effect. At the same time, the polymer has soft C-H segments. A large number of studies have shown that in this system, there are three phase regions of pure PEO phase, amorphous phase and salt-rich phase at room temperature, and the ion conduction mainly occurs in the highly elastic region of the amorphous phase. The crystalline phase form of the polymer electrolyte is generally only available in a few very specific components.
Pure solid polymer electrolyte is a complex formed by polymer matrix and doped salt, mainly polyether alkali metal salt complex, which does not contain solvent, and its conduction depends entirely on the ions in the polar polymer network. The polymer electrolyte obtained by simply mixing a PEO-based polymer body and a lithium salt is the most typical representative of this type of material, and is also known as the "first-generation polymer electrolyte". At 40~80℃, the conductivity of this type of electrolyte is between 10-8~10-4S/cm. Because the room temperature ionic conductivity is too low, this type of polymer electrolyte material is still difficult to practically apply. This is firstly because the high crystallinity of this type of electrolyte is not conducive to the conduction of ions in it, and the second reason is that the solubility of the amorphous phase PEO to the salt is very low.
Solid polymer electrolyte
In 1993, Bruce et al. reported on Science for the first time the crystal structure of the principle polymer electrolyte (PEO) 3: LiCF3SO3 determined by powder ray diffraction, pointing out that the PEO chain has a helical configuration parallel to the crystallographic b axis. Lithium ions combine with 5 oxygen atoms (three are from ethylene oxide, and every two adjacent CF3SO3 groups provide one more oxygen atom). The CF3SO3 group becomes a bridge connecting two lithium ions, forming a parallel chain that is entangled with the PEO main chain. There is no mutual cross-linked chain connection between the PEO chains, and the electrolyte can be regarded as an infinitely long cylindrical aggregate. However, this specific component is far from the actual polymer electrolyte with high ionic conductivity. Moreover, experiments have shown that as the polymer content increases from 3:1 to 6:1, the conductivity of the polymer electrolyte Will increase significantly. Therefore, the crystal structure of a polymer electrolyte with a ratio of 6:1 is more interesting. In 1999, the research group used the ab initio method to obtain a (PEO)6LiAsF6 complex that is closer to the actual polymer electrolyte by performing simulated annealing on the polymer electrolyte and fitting the entire powder-ray diffraction spectrum. . They found that the polymer chains in the polymer electrolyte with a composition of 3:1 form a helical shape, while the polymer chains in the mixture of a composition of 6:1 form a triple non-helical structure, and the two are embedded in each other. The sleeve forms a cylinder. Lithium ions are located in the cylinder and do not associate with anions.
It is generally believed that the alkali metal ions are first complexed with the polar groups on the polymer chain. Under the action of an electric field, with the thermal movement of the molecular segments in the high-elastic region, the alkali metal ions are dissociated from the polar groups. Then complex with other chain segments. Through this continuous complexation/decomplexation process, the directional migration of ions is realized. Therefore, the role of alkali metal ions and polymer segments plays a key role in the conduction of ions in the polymer electrolyte.
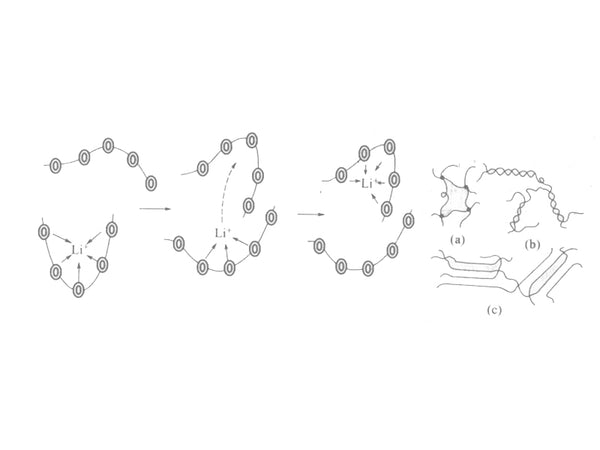
Illustration of ion transport in the amorphous region
To form a polymer electrolyte with high conductivity, the host polymer must have a strong electron donating atom or group, and its polar group should contain O, S, N, P and other atoms and cations that can provide lone pairs of electrons. Coordination bonds to offset the lattice energy of the salt. Secondly, the distance between the coordination centers should be appropriate to form multiple bonds with each cation to achieve good solubility. In addition, the polymer molecular chain segment should be sufficiently flexible, and the rotation resistance of the functional groups on the polymer should be as low as possible to facilitate the movement of cations. In addition to PEO, common polymer matrixes include polypropylene oxide (PPO), polymethyl methacrylate (PMMA), polyacrylonitrile (PAN) and polyvinylidene fluoride (PVdF).
Since the ion transport mainly occurs in the amorphous phase, the crystalline phase contributes little to the electrical conductivity. Therefore, the conductivity of the PEO/salt complex containing the crystalline phase at room temperature is very low, only 10-8S/cm, only when the temperature rises to crystallize When the phase is melted, the conductivity will be greatly improved, and most lithium-ion batteries work at room temperature, so by modifying the structure of the polymer or looking for a more suitable lithium salt, the development of a low glass transition The polymer electrolyte that is amorphous at temperature (Tg) and room temperature has become the focus of current research work. Indeed, many important research works have focused on improving room temperature conductivity through blending, copolymerization, comb/branching, and cross-linking networks. The common feature of these methods to improve conductivity is to reduce the crystallinity of the polymer or lower its glass transition temperature.
Commonly used polymer modification methods are chemical (such as copolymerization and crosslinking) and physical (such as blending and plasticization). Chemical cross-linking or covalent cross-linking is a process in which covalent bonds with polymer chains form a certain number of connection points through a chemical reaction. The formation of covalent cross-linking is an irreversible gel. In this kind of gel, the number of connection points does not necessarily change with changes in external conditions such as temperature, concentration, or pressure. In contrast, the gel network formed by physical crosslinking is called an entangled network. There are two main types of entanglement: one is the junction area, where the polymer chain cross-links a part of its own length; the other is the edge micelle method, where the polymer segments are oriented in a small area Arrange to form small crystal regions, and other weak interactions such as ion complexation will also help to form a physical gel network. Most gel polymer electrolytes are prepared in this way.
The use of EO and PO cross-linked block copolymers can increase the room temperature conductivity of the polymer electrolyte to 5×10-5S/cm. By linking PEO to the polysiloxane backbone to form a comb polymer, the polymer can be polymerized. The room temperature conductivity of the material electrolyte is increased to 2×10-4S/em. If PEO and PAMA are blended, and then LiClO4 is formed into a complex, a polymer electrolyte with room temperature conductivity higher than 10-4S/cm can be obtained. Someone blended styrene butadiene rubber and cyanide butadiene rubber to synthesize a two-phase electrolyte. Non-polar styrene butadiene rubber is the supporting phase to ensure that the electrolyte has good mechanical properties; polar cyanide butadiene rubber is the conductive phase, and lithium ions are conducted in the conductive phase, and the room temperature conductivity is as high as 10-3S/cm.
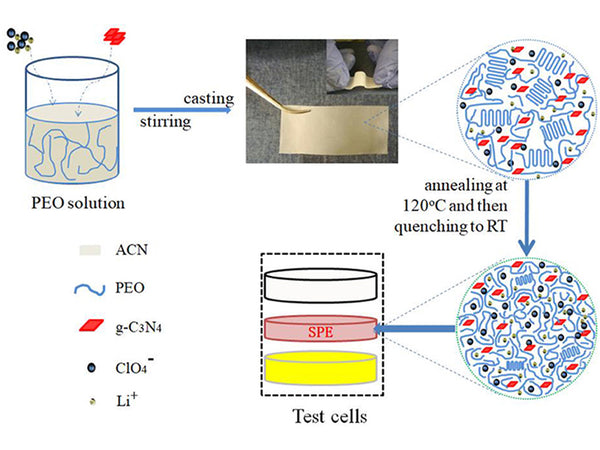
The flowchart of the preparation of the PEO-LiClO4-g-C3N4 solid composite polymer electrolyte film.
Since the concept of polymer electrolyte formed by PEO and lithium salt was proposed, people have conducted extensive research on the ion conduction mechanism. In this system, lithium ions associate with the ethylene oxide on PEO and move with the polymer segment, but the electron donating ability of ethylene oxide is too strong, and the lithium ion cannot effectively move with the polymer segment, which reduces the polymerization. Considering the electron-donating ability of the functional group, the aliphatic polycarbonate (Mw=50,000) is selected as the polymer body, and the ionic conductivity of the polymer electrolyte prepared with LiTFSI as the lithium salt increases as the glass transition temperature of the sample decreases. And at 0.8mol (relative to the monomer unit of the polymer), the highest conductivity is 0.5×10-4S/cm (at 20°C).
To meet practical requirements, polymer electrolytes must not only have high room temperature conductivity, but also high lithium ion mobility. When the battery is working, the concentration polarization between the two poles of the battery due to the migration of anions and cations in the opposite direction improves the performance of the battery. A common practice is to choose a lithium salt with a larger anion, which will not only reduce the dissociation energy of the salt and increase the concentration of lithium ions in the electrolyte, but also the larger anion will be more restricted by the polymer body to its movement Therefore, the lithium ion migration number of the polymer electrolyte can be increased. For example, by using the restriction effect of boron atoms on the movement of anions, a polymer electrolyte with a lithium ion migration number greater than 0.5 can be obtained through the reaction between polyoxypropylene oligomer and polyboronbicyclononane.
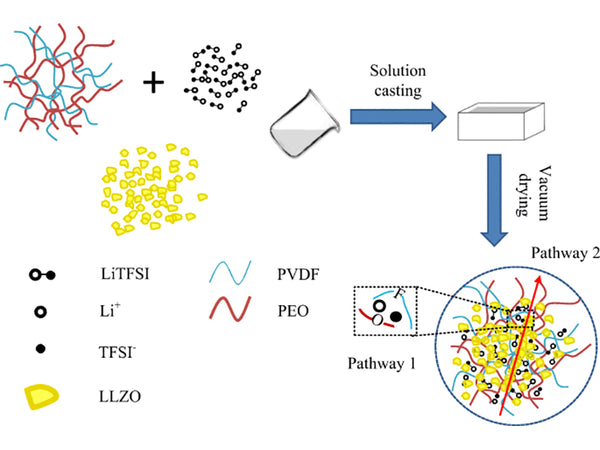
Composite solid electrolyte with LLZO added to PEO/PVDF matrix
To increase the migration number of cations, some researchers covalently bond anions to the backbone of the polymer, allowing only lithium ions to move with the polymer chain segments to obtain a single-ion conductive polymer electrolyte. These polymer electrolytes are called polyelectrolytes (polyelectrolytes). Compared with ordinary polymer electrolytes, polyelectrolytes have a unique advantage when used in batteries. They are not susceptible to the formation of high or low salt concentration potential barriers at the electrode/electrolyte interface during cycling. The anions are effectively fixed on the polymer backbone, so all the ionic conductivity of the polyelectrolyte is the contribution of cation migration. For example, fix the anion on the polymer chain segment so that it cannot move freely in the electrolyte and increase the lithium ion migration number of the polymer electrolyte. However, the room temperature conductivity of such an electrolyte is generally below 10-6S/cm, which is higher than the double The conductivity of ion-conducting polymer electrolytes is 1 to 2 orders of magnitude lower.
Using boroxine ring and oligoether side chain to bind anions, a polymer electrolyte with high lithium ion migration number and room temperature ion conductivity (10-5S/cm) can be obtained. For example, the ionic conductivity of a polymer electrolyte composed of polyethylene glycol methacrylate and polyethylene glycol borate can reach 10-4S/cm at 30°C. The ionic conductivity of the polymer electrolyte composed of methoxy polyethylene glycol borate, methacryloyl polyethylene glycol borate and LiTFSI reaches 3.8×10-4S/cm at 20°C, which is high The ionic conductivity of the traditional PEO electrolyte. The lithium ion migration number of this polymer electrolyte increases with the increase of the borate content in the polymer. The maximum lithium ion migration number at 20°C is close to 1. NMR measurement of 11B showed that with the addition of lithium salt, the chemical shift of this peak changed from 10.8 to 14.4. This shows that the electron density of borate is very high, and borate can bind the anions in the lithium salt. Accordingly, by increasing the content of borate in the polymer electrolyte, a single-ion conductive polymer electrolyte can be obtained.
Glass and ceramic materials conduct electricity through a fast ion conduction mechanism, in which lithium ions basically move in a static frame. On the contrary, in the polymer system, the movement of lithium ions is similar to the movement in the liquid electrolyte, that is, the movement of lithium ions is mediated by the dynamics of the polymer body, which limits the conductivity value to a relatively high value. In the low range, the movement of lithium ions has only a small contribution to the total conductivity of the electrolyte (low migration number). A new polymer electrolyte is prepared by doping lithium ions in plastic crystals. Due to the disorder of rotation and the existence of lattice vacancies, this type of polymer electrolyte material has the conductive properties of fast ions, and the conductivity reaches 2×10-4S/cm at 60°C.