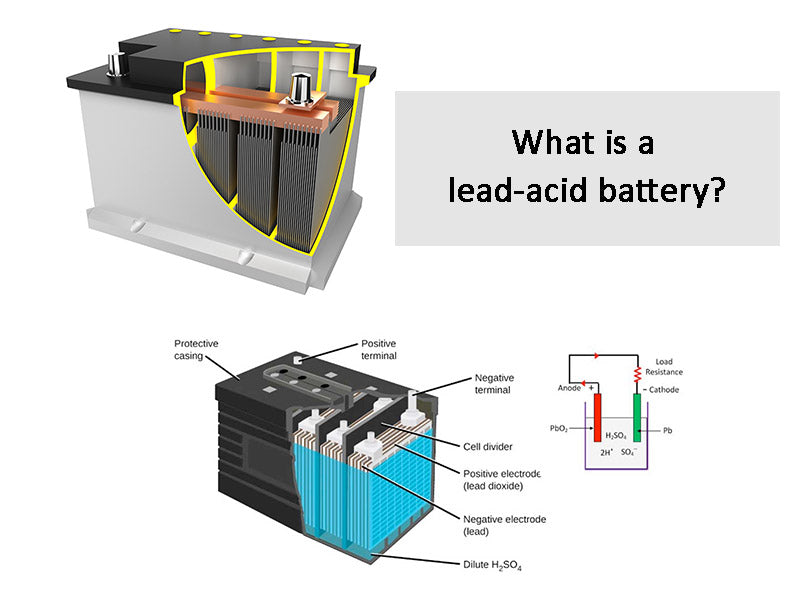
A lead-acid battery is a kind of battery that uses lead compound (lead dioxide) as the positive electrode material, metal lead as the negative electrode material, and sulfuric acid solution as the electrolyte, and stores and releases electrical energy through the chemical reaction of lead and sulfuric acid. A typical lead-acid battery, regardless of its application, has the structure listed in Figure 1.

Figure 1 - Basic structure of lead-acid battery
①The positive and negative terminals are made of lead and are used to connect external power-consuming devices.
②Vent plugs are equipped with one for each set of electrodes to replace distilled/deionized water when necessary, and to be used as an escape channel for the gas generated in the battery.
③The connecting piece is made of lead, which is used to form the electrical connection between the electrode plates of the same polarity and provide the electrical connection between the electrodes with a distance from each other.
④The battery box and box cover were made of bakelite before, but now polypropylene or polymer is generally used.
⑤Sulfuric acid solution The electrolyte in the battery.
⑥ Electrode separators are generally integrated with the battery box and use the same material to provide chemical and electrical isolation between electrodes. The electrode separators are connected in series to increase the final voltage provided by the battery.
⑦ Electrode plate separators are made of PVC and other porous materials to avoid physical contact between adjacent circuit boards, but at the same time allow free movement of ions in the electrolyte.
⑧ The negative electrode plate is composed of metal lead grid, and the surface is coated with lead dioxide paste.
⑨ The positive electrode plate consists of a metal lead plate.
⑩The battery electrode consists of a series of positive and negative electrode plates placed in sequence and separated from each other by separators, and the electrode plates of the same polarity are connected on the electrical appliance.
When a lead-acid battery supplies power to an external device, several chemical reactions occur simultaneously. The reduction reaction of lead dioxide (PbO2) into lead sulfate (PbSO4) occurs at the positive electrode plate (cathode); the oxidation reaction occurs at the negative electrode plate (anode), and the metal lead becomes lead sulfate. The electrolyte (sulfuric acid) provides sulfate ions for the above two semi-electrolytic reactions, acting as a chemical bridge between the two reactions. Every time an electron is generated at the anode, an electron is lost at the cathode, and the reaction equation is
Anode: Pb(s)+SO42-(aq)→PbSO4(s)+2e-
Cathode: PbO2(s)+SO42-(aq)+4H++2e-→PbSO4(s)+2H2O(l)
Completely reactive: Pb(s)+PbO2(s)+2H2SO4(aq)→2PbSO4(s)+2H2O(l)
The battery can be repeatedly charged and discharged for hundreds of times and still maintain good performance. However, since the lead oxide electrode plate is gradually polluted by lead sulfate, it may eventually lead to the chemical reaction not taking place at the lead oxide electrode plate. Finally, due to heavy contamination, the battery may not be able to be recharged again. At this time, the battery becomes a "waste lead-acid battery".
Lead-acid batteries have a variety of uses, and the voltage, size and quality used are also different. The lighter ones are constant voltage batteries with a weight of only 2kg; the heavy ones are industrial batteries, which can reach more than 2t. According to different uses, batteries can be divided into the following categories.
①Automobile battery refers to the main energy used by vehicles such as cars, trucks, tractors, motorcycles, motor boats, and airplanes when starting engines, lighting and igniting.
②Ordinary battery refers to portable tools and batteries used in equipment, indoor alarm systems and emergency lighting.
④Power battery refers to the battery used in forklifts, golf carts, luggage transport vehicles at airports, wheelchairs, electric vehicles and passenger cars and other means of transporting goods or people.
⑤Special battery refers to the battery that is dedicated or combined with electrical and electronic circuits in some scientific, medical or military applications.
Ignition lead-acid batteries account for the largest percentage of all lead-acid battery uses. At present, there are many manufacturers in China's automobile and motorcycle industries, and there is no uniform industry standard for the type of battery used. Many large companies have their own corporate standards, resulting in a variety of battery types and sizes. Vehicles with a transport capacity of less than 3t and batteries for cars generally have only 6 lead plates, and the mass is 15~20kg.
The picture below shows the waste lead-acid battery after the car is naturally scrapped.
Lead-acid battery is currently the largest and most widely used type of battery in the world. Of the world's annual lead production, lead-acid batteries in automobiles, industrial facilities and portable tools often account for 75% of the world's total lead consumption. Developed countries in the world attach great importance to the recovery of secondary lead. In 1999, the total amount of lead in Western countries was 4.896 million tons, of which the output of secondary lead was 2.846 million tons, accounting for 58.13% of the total. The total annual output in the United States is 1.422 million tons, of which the production of secondary lead is 1.083 million tons, accounting for 76.2% of the total. The proportion of secondary lead production in France, Germany, Sweden, Italy, Japan and other countries all exceeds 50%. In some countries, such as Brazil, Spain and Thailand, 100% of lead consumption depends on recycled lead.
At present, more than 85% of China's recycled lead raw materials come from waste lead-acid batteries, and 50% of the lead consumed by the battery industry is recycled lead. Therefore, the recovery of secondary lead from waste batteries occupies a very important position in China's lead industry.