
main content:
With the development of modern science and technology, electronic and electrical equipment are changing to miniaturization and light weight. The increase in the use of portable electrical appliances has also led to the rapid development of the battery industry. As the smallest battery among the batteries, the consumption of the button battery has also increased accordingly. Although button batteries only account for 0.5% of the total battery consumption, due to the high mercury content in them, these batteries will cause great harm to the environment once they are discarded. Therefore, the recycling of waste button batteries is an important issue in the disposal of waste batteries.
Button battery generally refers to a small battery in the shape of a coin or a button. It is widely used in portable electronic products such as flashlights, electronic cameras, stroboscopes, electronic watches, electronic toys, hearing aids and various meters. . Button batteries mainly include alkaline zinc-manganese button batteries, silver oxide button batteries, mercury oxide button batteries, zinc-air button batteries and lithium batteries (button type).
1. Alkaline zinc manganese button battery

Figure 1 - Construction of a generic alkaline zinc-manganese coin cell battery
The alkaline zinc-manganese button battery uses powdered zinc as the anode and manganese dioxide as the cathode. Its electrolyte is potassium hydroxide, so it is called an alkaline zinc-manganese button battery. The most common type of alkaline zinc-manganese coin cell battery is LR44. Since the zinc negative electrode is formed by zinc powder or colloid, instead of using a plate, the utilization rate of zinc is higher than that of zinc-carbon batteries, the discharge time is longer, and it is more suitable for high-load and high-power discharge. The discharge reaction is as follows.
2MnO2 +2H2O+Zn→2MnOOH+Zn(OH)2
The structure of the general alkaline zinc-manganese button battery is shown in Figure 1.
Since the zinc negative electrode is formed by amalgamation treatment with mercury after the zinc is melted, powdered or micronized. While the surface area of the zinc negative electrode is increased and the utilization rate is improved, the mercury content in the alkaline zinc-manganese button battery is correspondingly higher than that of other batteries. If they cannot be recycled and disposed of in a centralized manner for a long time, and they are left to be thrown around, it will inevitably cause greater mercury pollution and bring harm to the environment and society.
2. Silver oxide button battery
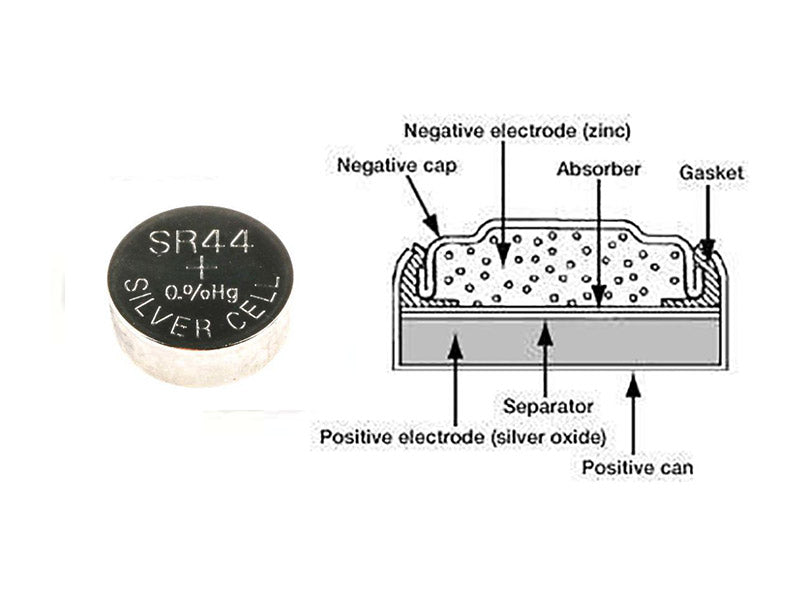
The positive electrode of the silver oxide button battery is a cell formed by pressing silver oxide and graphite (sometimes manganese dioxide is added to the positive electrode), which is closely connected to the nickel-plated steel cylinder, and the negative electrode is zinc powder (amalgam), potassium hydroxide or sodium hydroxide aqueous solution containing saturated zincate is used as electrolyte, and the contact surface with zinc is usually a steel cylinder plated with Sn or plated with Cu. The content of zinc powder in the zinc amalgam is 2% to 15%. The casing of the battery is typically composed of layers of copper, tin, stainless steel, nickel-plated steel, or nickel. The discharge reaction of the silver oxide coin cell is as follows.
AgO+H2O+Zn→Ag+Zn(OH)2
Figure 2 shows the construction of a silver oxide coin cell battery using the SR44 type as an example.
The discharge voltage of silver oxide batteries is very stable and is one of the high-performance batteries. Since the positive electrode active material uses a silver compound, the cost is relatively high and the recycling value is relatively high.
Silver oxide batteries have been widely used as power sources for cameras and desktop electronic computers. In recent years, due to technological progress, they have been gradually replaced by lithium batteries and alkaline button batteries. At present, it is mainly used as a power source for quartz watches. Although the output is small, it also affects environmental protection.
3. Mercury oxide button battery
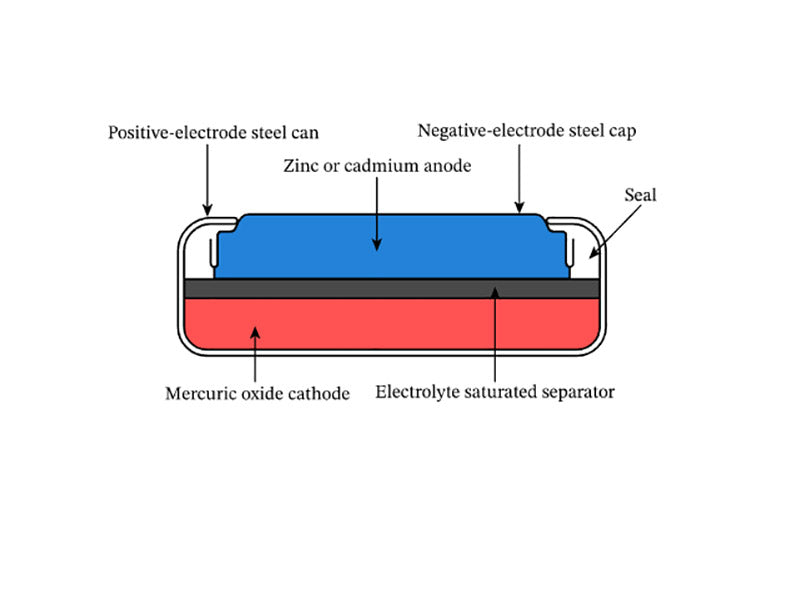
The mercury oxide button battery is sealed with a steel container, and the positive electrode is a cell formed by pressing mercury oxide and graphite, which is closely connected to the nickel-plated steel cylinder. The electrolyte is potassium hydroxide or sodium hydroxide solution, and the contact surface with zinc is usually a steel cylinder plated with Sn or plated with Cu. Some varieties use brocade instead of zinc for some specific purposes, such as data logging of natural gas and oil wells, and telemetry and alarm systems for engines and other heat sources. The discharge reaction of a mercury oxide coin cell battery is as follows.
HgO+H2O+Zn→Hg+Zn(OH)2
Figure 3 shows the structure of a mercury oxide coin cell battery.
Mercury oxide button battery has the characteristics of flat discharge, stable, high capacity and long discharge time. Because the mercury content is as high as 30%~50%, it is highly dangerous to the environment and human body, and also has a high recovery value.
4. Zinc-air button battery
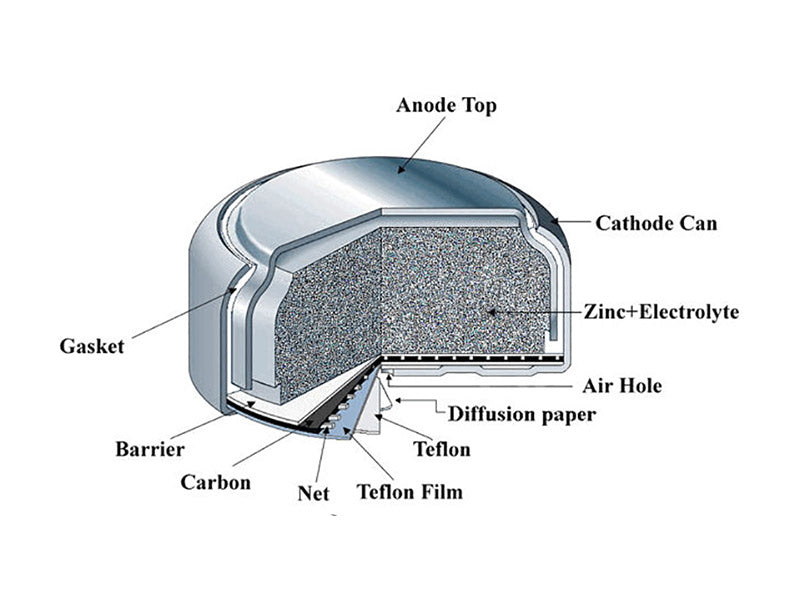
Zinc-air coin cells generate electricity directly from the oxygen in the air. Oxygen in the air diffuses into the cell and is then used as a reactant for the cathode. The cathode consists of loose zinc powder mixed with an electrolyte (sometimes with an adhesive). The electrolyte is about 30% potassium hydroxide solution. Figure 4 shows the structure of a zinc-air coin cell battery.
5. Button-type lithium battery
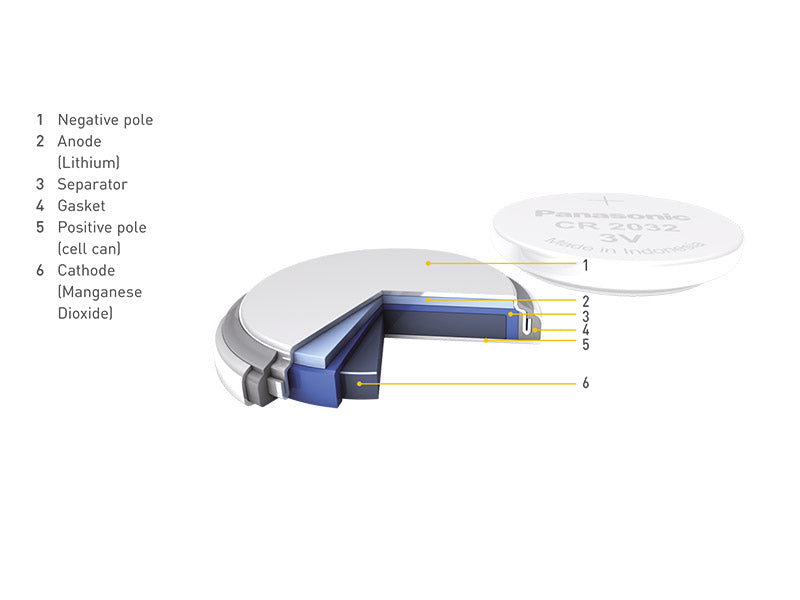
Button-type lithium batteries mainly include lithium-manganese dioxide button batteries and lithium-ion button 135 button batteries.
Lithium-manganese dioxide button battery is a kind of disposable battery with lithium as anode, manganese dioxide as cathode, and organic electrolyte. The main features of this kind of battery are high battery voltage, rated voltage is 3V (2 times that of general alkaline batteries); termination discharge voltage is 2V; specific energy is large; discharge voltage is stable and reliable;
It has good storage performance (storage time of more than 3 years), low self-discharge rate (annual self-discharge rate <2%); operating temperature range -20~60℃.
Lithium-manganese dioxide button batteries are small in size, generally 12.5~24.5mm in diameter and 1.6~5.0mm in height. Commonly used in clocks, calculators, electronic notepads, cameras, hearing aids, electronic game machines, IC cards, backup power supplies, etc.
Figure 4 shows the construction of a lithium-manganese dioxide coin cell battery.
Lithium-ion button batteries are rechargeable batteries, and they account for a small number of lithium-ion batteries and are not very common.
The elements contained in button-type lithium batteries are quite different from other button batteries, and basically do not contain harmful components such as mercury and cadmium, so they can be treated together in lithium batteries.
In addition to button-type lithium batteries, the other four button batteries contain mercury, nickel, cadmium and other toxic and harmful components. These toxic and harmful substances can pollute soil, water or air in various ways. Eating or drinking contaminated food or water, inhaling contaminated air, or directly contacting and absorbing through the skin can harm human health.
Although mercury-free zinc powder has been applied in recent years, making alkaline batteries into a green battery, China is also gradually making regulations on the range of mercury content in various types of batteries, promoting the production of mercury-free batteries, and striving to control the environmental pollution of batteries from the source, the mercury content in button batteries still accounts for a large proportion . As for now, since there are still a large number of old-fashioned battery manufacturers in China, it is difficult to completely achieve mercury-free in a short period of time. Therefore, for button batteries (except button-type lithium batteries), it is necessary to carry out classified collection and recycling.