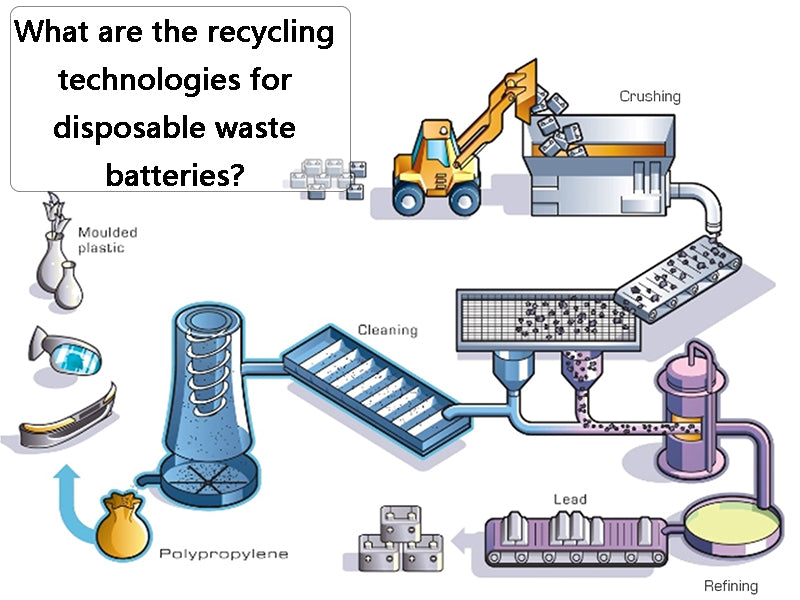
main content:
Recycling waste batteries is not only conducive to saving resources, but also improving the environment.
1. Manual sorting and recycling technology

Sort the recycled waste dry batteries first, cut the dry batteries one by one with a simple machine in a work cabinet with a negative pressure and purification device, manually separate the plastic covers, and then send them to the plastic factory for recycling; the iron shell is sent to the smelter for recycling ; Carbon rods with copper caps are separated and recovered copper and carbon rods; Zinc skins are washed and sent to an electric furnace to be re-melted into zinc ingots; The remaining mixture of manganese dioxide and manganite (MnOOH) is sent to a rotary kiln for calcination , Manganese dioxide is obtained after dehydration, which can be used as a chemical raw material. In addition, the black filler in the battery can also be immersed in water, left to settle, and the supernatant is filtered, evaporated and crystallized to prepare ammonium chloride; the carbon black and electric paste in the battery can also be used to prepare fertilizer, It can promote the photosynthesis of rice seedlings and prevent the dead seedlings caused by zinc deficiency. This fertilizer can also be used for field spraying of rice or as a fertilizer for crops such as sugar cane and sweet potato, but its dosage should be selected according to different crops.
2. Wet recycling technology
Wet recycling is based on the principle that zinc, manganese dioxide, etc. are soluble in acid, so that zinc, manganese dioxide and acid in dry batteries enter the solution, and the solution is purified and electrolyzed to produce metal zinc, manganese dioxide or chemical products. (such as lithopone, zinc oxide, etc.) and fertilizers. The methods used are direct leaching and roasting leaching. Because the waste dry battery contains a variety of substances, the wet recycling process is long, and the recovered electrolyte contains heavy metals such as mercury, cadmium, and zinc, and the energy consumption is also high.
The specific method is to classify the waste dry batteries, crush them and place them in a leaching tank, add dilute sulfuric acid (100~120g/L) for leaching, all zinc and its compounds are dissolved in the sulfuric acid solution, filtered, and the filtrate is ZnSO4. Metallic zinc is prepared by plot method or zinc sulfate is prepared by concentrated crystallization method; the filter residue is washed and filtered, and the waste water is treated and discharged up to the standard. After the copper cap and iron sheet are separated from the filter residue, the remaining sludge is mainly manganese dioxide and manganite, which can be The pyrolusite powder is used to prepare an oxidizing liquid, and it can also be calcined into manganese dioxide as a chemical raw material. In places with sufficient power, manganese sludge can be leached, purified, and electrolyzed to produce electrolytic manganese. The wet recycling technology is shown in Figure 1.
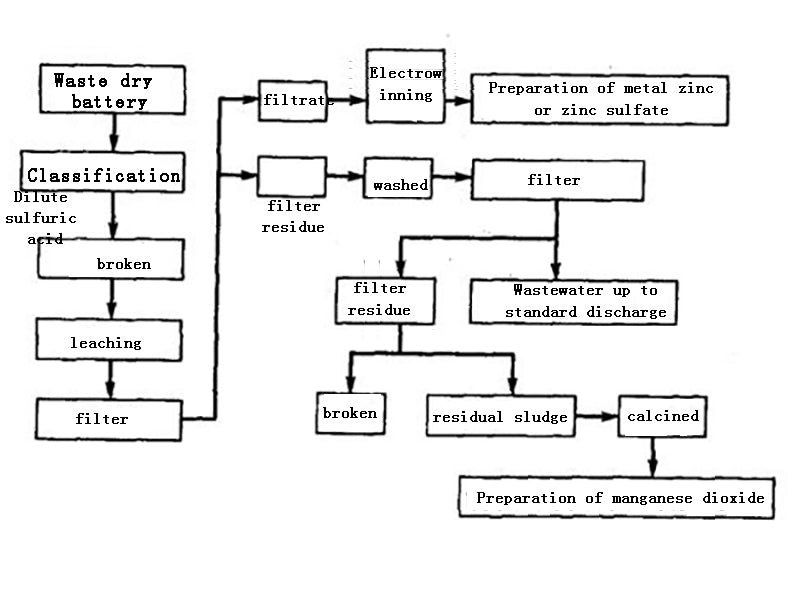
Figure 1 Wet recycling technology
This method is economically feasible when the chloride ion concentration in the leachate is less than 100 mg/L.
A "wet treatment" plant is under construction in the suburbs of Magdeburg, Germany. Except for lead batteries, all kinds of batteries are dissolved in sulfuric acid, and then various metals are extracted from the solution with the help of ionic resins. The raw materials obtained in this way are purer than the heat treatment method, so they are more expensive in the market, and the batteries contain 95% of the various substances can be extracted.
3. Dry recycling technology
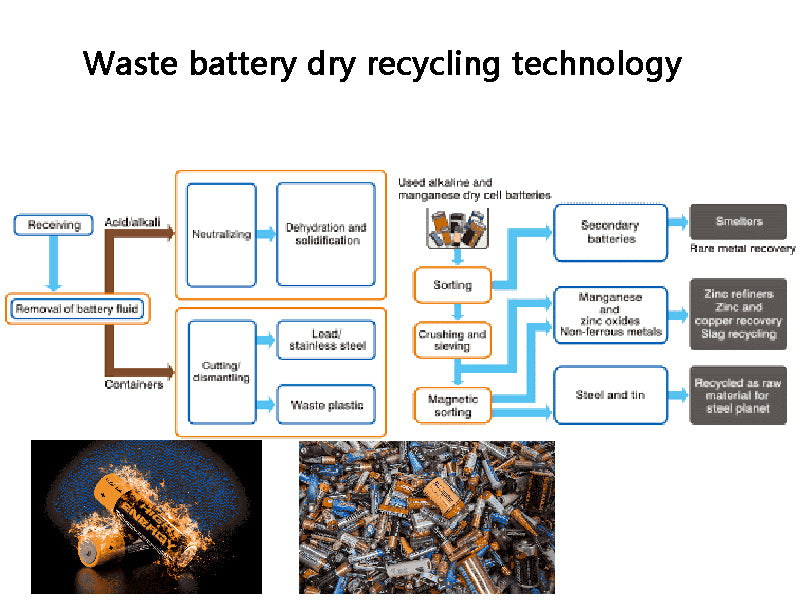
Dry recycling of dry batteries is to oxidize, reduce, decompose, volatilize and condense the metals and their compounds in dry batteries at high temperatures. This method is further divided into traditional atmospheric metallurgy and vacuum metallurgy.
The waste dry batteries are sorted, screened, crushed, sent to the roasting furnace, and roasted at 600 ° C. The mercury-containing waste gas is collected by a cyclone dust collector or a dry electrostatic precipitator, or the mercury is recovered by air cooling or condenser cooling. Usually mercury vapor starts to condense at 100~150°C, and the condensed metal mercury particles on the inner wall are regularly washed and recovered with water, refined into mercury with a purity of 99.9%, and sold. The roasting residue is transferred to the melting furnace or rotary kiln. At the high temperature of 1100~1300℃, zinc and zinc chloride are oxidized to zinc oxide, which is discharged with the flue gas, and the zinc oxide is recovered by a cyclone dust collector or a bag filter. Residual manganese dioxide, manganese stone and iron enter the residue to further recover iron, manganese or prepare ferromanganese alloy.
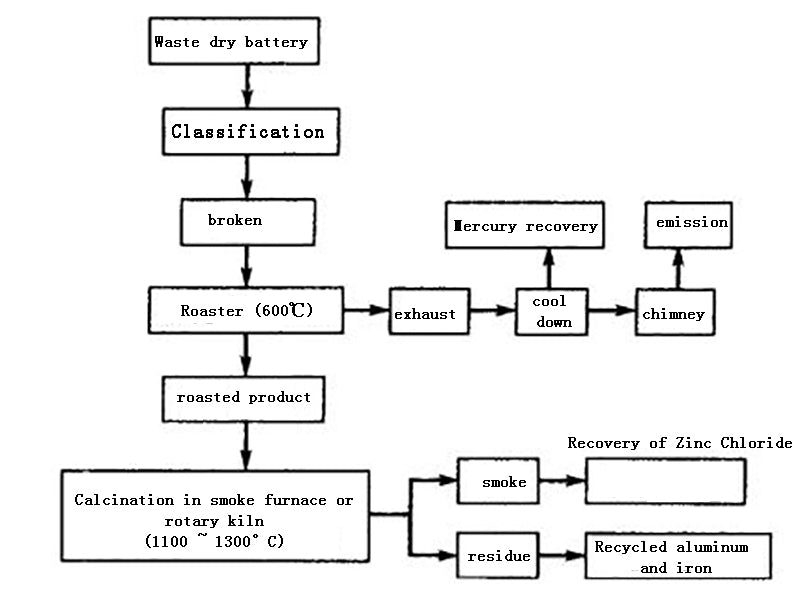
Figure 2 Block diagram of dry recycling technology
Compared with the wet method, the dry method can recover more heavy metals such as mercury, nickel, and zinc. Combustible substances in dry batteries such as carbon rods, plastics, paper, and graphite are removed by combustion. The roasting product contains heavy metals such as manganese and iron. It can be used as a metallurgical intermediate product. Since the atmospheric pressure metallurgy for the treatment of waste dry batteries is carried out in the atmosphere, the air participates in the reaction, causing secondary pollution and high energy consumption. Therefore, there are many problems and difficulties in industrial applications as in wet processes.
Vacuum metallurgy is based on the fact that each part of the waste dry battery has different vapor pressures at the same temperature, and they are separated and condensed at different temperatures in a vacuum to realize the comprehensive recovery and utilization of dry batteries. At present, there are few researches on the vacuum metallurgical recovery method of waste dry batteries, but it is basically pollution-free or less polluted compared with the wet method and atmospheric metallurgical method, and the consumption is low. method (tons of refined zinc) (6~10)×106kJ, electrolysis method (10.8~12.6)×106kJ, vacuum metallurgy method is less than 3.6×106kJ], the comprehensive recovery rate of various useful components is high, and the benefit is good, it is a very promising approach.
The Swiss Bartlek company grinds the waste batteries, and then sends them to the furnace for heating. Mercury is volatilized at a lower temperature, and zinc is evaporated at a higher temperature. The iron and manganese are fused to become the ferromanganese alloy required for steelmaking. The factory can process 2000t of waste batteries a year, and can obtain 780t of ferromanganese alloy, 400t of zinc alloy and 3t of mercury. Another plant extracts iron directly from batteries and sells a mixture of metals such as manganese oxide, zinc oxide, copper oxide and nickel oxide directly as scrap metal.
In 1984, Japan's Nomura Kogyo Co., Ltd. successfully developed a complete set of experimental equipment for the recycling of mercury-containing wastes in Hokkaido, and in 1985, a 6000t/a recycling equipment was built. The technological process is to heat the dry battery to 600~800℃ in a rotary kiln to vaporize the mercury, and then send it to the condenser to condense it into powder. Zinc, manganese, bell, iron and other metals contained in the slag of the rotary kiln are first separated from iron and manganese by a magnetic separator, and then used as primary raw materials respectively. In 1995, TDK Corporation and Nomura Kogyo Co., Ltd. made bold reforms to the regeneration process. After crushing the waste batteries, they were heated at high temperature to remove impurities, and the metal elements were oxidized to become the raw materials for the manufacture of iron oxide. Since the separation process has been simplified, the cost has been greatly reduced, and the efficiency has been further improved through classification and regeneration.
The vacuum heat treatment method developed by the German company Alte has a lower cost, but it first needs to sort out the nickel-cadmium batteries from the waste batteries, and then heat the waste batteries in a vacuum device, where the mercury evaporates quickly and can be recovered; then the remaining The raw material is ground, the metal iron is extracted with a magnet, and nickel and manganese are extracted from the remaining powder. This method costs less than 1500 Deutsche Marks (about 750 Euros) to process 1 ton of waste batteries.
4.Dry and wet recycling process
After the waste dry batteries are screened, classified, crushed, and magnetically separated to remove iron, they are placed in an electric heating rotary kiln. Since the batteries contain acetylene black and graphite, their content is about twice the theoretical amount of carbon required to reduce MnO2 to MnO. Therefore, the reducing agent may not be supplemented during the battery baking. Usually, the roasting temperature is controlled at about 850°C, and should not exceed 900°C. Because at 850 ℃, the zinc shell of the battery will enter the flue gas in the form of vapor, and the zinc-containing flue gas will be cooled, and the zinc will be recovered by a bag filter. After the roasted product is cooled, impurities such as copper caps and carbon rods are removed. The ratio of liquid to solid is 5:1, and sulfuric acid solution with a concentration of <200g/L is used as the solvent. The leaching temperature is kept at 800°C and the leaching time is 1h. Under this condition, all the residual zinc enters the solution, and the leaching rate of manganese (MnO) is greater than 95%. The composition of the leaching solution is shown in Table 1.
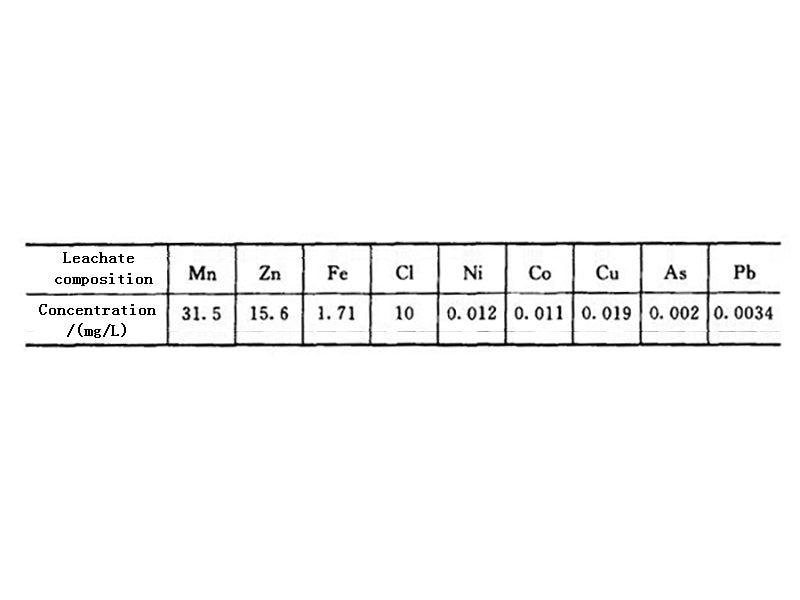
Table 1 Composition of sulfuric acid leaching solution of roasting residue in electric heating rotary kiln
It can be seen from Table 1 that the composition of the sulfuric acid leaching solution of the dry battery roasting residue is relatively complex, and the impurity content is greater than the allowable value of the impurity content in the zinc hydrometallurgy process. Therefore, before electrowinning, the solution must be purified to remove impurities such as iron, copper, cobalt, and nickel. The simultaneous recovery of zinc and manganese by electrowinning is a double electrowinning process. The electrowinning conditions of the anode and cathode are different, so it is very important to adjust the working state of the two electrodes reasonably. For example, for electrolytic manganese dioxide, the current density is preferably below 1A/m², while the current density for electrolytic zinc is preferably up to 1000A/m². This contradiction is usually solved by adjusting the electrode area and selecting electrodes of different materials; The influence of the manganese dioxide is also different, and the temperature has a great influence on the manganese dioxide, while the influence on the zinc is not obvious. Therefore, it is more appropriate to control the temperature at 85~90℃. In addition, it is also necessary to select the appropriate acidity and try to remove chloride ions in the solution to improve the efficiency of zinc-manganese double electrowinning. Someone once made an economical analysis on the regeneration process of "roasting-electrowinning method", and the results show that this method is technically and economically feasible.
The recycling process of waste dry batteries, whether it is dry, wet (see, Figure 3) or semi-dry and semi-wet, is a process with high technical content and potential secondary pollution risks. When determining the technical route, it is necessary to consider not only the economic feasibility, but also the environmental protection. Therefore, in the process of resource regeneration of waste batteries, it is necessary to strictly prevent the secondary pollution of the environment caused by the "three wastes" generated in the process.
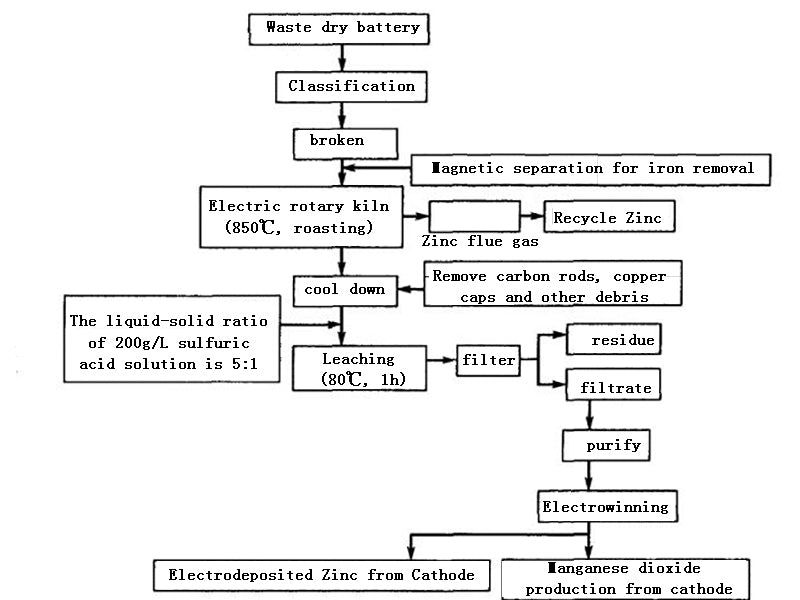
Figure 3 Dry and wet recycling technologies