What are the characteristics of the sol-gel method
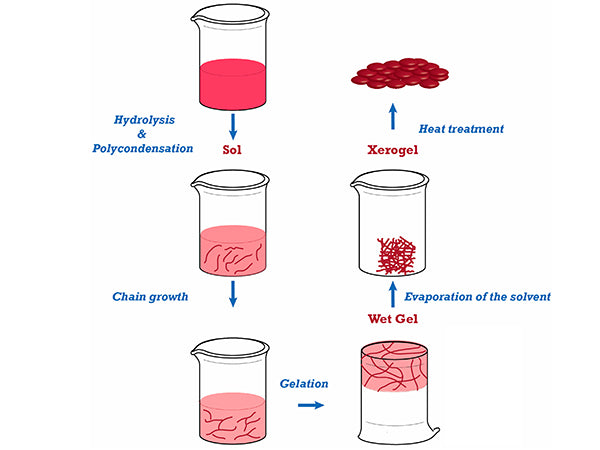
The sol-gel method is the sol-gel method. It is a new process for preparing materials developed in the 1960s. This method is a method in which metal alkoxides or inorganic salts are solidified through solutions, sols, and gels, and then the gels are converted into oxides by low-temperature heat treatment. The preparation process includes sol preparation, sol-gel conversion and gel drying, of which gel preparation and drying are the key. When metal alkoxide is used to prepare oxide powder, the sol is first prepared, and then the alkoxide is hydrolyzed and polymerized to form a gel, followed by aging, drying, and heat treatment to finally obtain the product. If an inorganic salt sol-gel is used to prepare the oxide powder, the colloidal chemistry theory is used to first solize the particles, then perform the sol-gel conversion, and then undergo aging, drying, and heat treatment operations.
The characteristics of the sol-gel method are: a lower reaction temperature, generally room temperature or slightly higher temperature, most organic active molecules can be introduced into the system and maintain their physical and chemical properties; because the reaction starts from a solution, The ratio of each component is easy to control and is uniform at the molecular level; because it does not involve high-temperature reactions, the introduction of impurities can be avoided, and the purity of the final product can be ensured; films, fibers, or fibers can be prepared at different stages of the reaction as required. Block functional materials. The disadvantage is that some alkoxides are harmful to the human body and are more expensive. At the same time, the processing cycle of this law is too long.
Therefore, the sol-gel method has good chemical uniformity, narrow particle size distribution, high purity, small particle size, easy control of the reaction, low synthesis temperature, large specific surface area of the obtained material particles, and can accommodate insoluble components or does not precipitate. Sexual components and other advantages.
The process of the sol-gel method is as follows:
(1) Preparation of sol
The obtaining of sol is divided into two types: inorganic method and organic method. In the inorganic method, the formation of sol is mainly completed by the hydrolysis of inorganic salts, and the reaction expression is as follows:
Mn+ +nH2O--->M(OH)n+nH+
In the organic approach, organic alkoxides are used as raw materials to prepare sols through hydrolysis and polycondensation reactions. The reaction formula is expressed as:
M(OR)n, +χH20--->M(OR)n-χ(OH)χ+χROH (hydrolysis)
2M(OR)n-χ(OH)χ--->[M(OR)n-χ(OH)χ-1]2+H2O (condensation)
The process is as follows.
Alkoxide-hydrolysis, sol-polycondensation, gel-heat drying, xerogel-calcined-product.
(2) Sol-gel conversion
The sol contains a large amount of water. During the gelation process, the gelation is achieved by changing the pH value of the solution or heating and dehydrating. In the process of sol-gel conversion, hydrolysis and polycondensation are not two isolated processes. Once the alkoxide is hydrolyzed, water-loss polycondensation and alcohol-free polycondensation also proceed almost simultaneously, and M-O-M bonds are formed to form a sol system.
-M-OH+HO-M--->-M-O-M-+H2O
-M-OH+HO-M--->-M-O-M-+ROH
At room temperature, alkoxides are generally not miscible with water, so alcohol or other organic solvents are needed as co-solvents, and water and catalysts are added to the organic solution of alkoxides (alcohol salt hydrolysis generally requires a certain catalyst, commonly used acid and alkali catalysts , Generally hydrochloric acid or ammonia).
The hydrolysis reaction of metal alkoxide is related to many factors such as the catalyst, the type of alkoxide, the molar ratio of water and alkoxide, the type and amount of co-solvent, and the hydrolysis temperature. To study and grasp the influence of these factors on the hydrolysis is to control the hydrolysis. The key to the process.
(3) Drying of the gel
The large amount of solvent contained in the wet gel needs to be dried to remove it before the heat treatment, thereby obtaining a dry gel, and the generation of pores is minimized during the heat treatment. During this process, the gel structure changes greatly, so the heating process and the final temperature of the heat treatment have a greater impact on the material properties. As a result of the hydrolysis and polycondensation, the initial particles of the sol are formed. The initial particles gradually grow up, connect to form chains, and finally form a three-dimensional network structure to obtain a gel. The gel after polycondensation is called wet gel, and the drying process is to remove the physically adsorbed water and organic solvents and chemically adsorbed hydroxyl or alkyl residues in the wet gel. The drying process is mainly to control the drying speed, too fast will cause the gel to crack and break. The dry gel is treated at a constant temperature at a selected temperature to obtain a dense product.
In the field of lithium-ion batteries, the sol-gel method is mainly used to prepare cathode materials lithium cobalt oxide, lithium nickel oxide, lithium manganate, vanadium oxide, and anode material tin oxides.
Dissolve an appropriate amount of nickel nitrate, cobalt nitrate, manganese acetate and lithium nitrate in molar ratio Li/M=1.15, Co/Ni/Mn=1:1:1:1 in a certain amount of water and mix well, add citric acid while stirring. Make it form a uniform and transparent solution, heat and evaporate part of the water, and finally form a pink sol, which is dried at 120°C to form a gel. After grinding the gel finely, the precursor is obtained. The precursor is placed in a porcelain firing boat and placed in a microwave muffle furnace, heated at 1℃·min-1, kept at 950℃ for 8h, and cooled at 1℃/min. Target product.
The preparation of lithium cobaltate by the sol-gel method generally involves first dissolving the cobalt salt, and then gradually adjusting the pH value of the system with lithium hydroxide and ammonia water to form a gel. In this process, pH control is more important. If it is not well controlled, it is difficult to form precipitation, so it is also called precipitation method or co-precipitation method.
In order to well control the particle size and uniformity, organic acids can be added as carriers, such as oxalic acid, tartaric acid, citric acid, polyacrylic acid, etc. In the formed gel, since the oxygen atoms in the organic acid are combined with cobalt and lithium ions, not only the lithium and cobalt are mixed at the molecular level, but also the distribution of powder particles in the nanometer range can be ensured.
The sol-gel method can obtain lithium cobalt oxide with good crystallinity at a lower synthesis temperature, and it does not require long-term heating like a solid phase reaction. The reversible capacity of the obtained lithium cobalt oxide can reach more than 150mA·h/g, and the reversible capacity of the solid-phase reaction is generally about 120mA·h/g. With metallic lithium as the reference electrode, the material obtained by the sol-gel method has a capacity of more than 140mA·h/g after 10 cycles.
Like the preparation of lithium cobalt oxide, lithium hydroxide, ammonia water and nickel salt can also be used to produce lithium nickel oxide. The gel is obtained by this method, and the nickel hydroxide reacts with the lithium hydroxide to obtain a nano-scale mixture, and then the mixture is hot buried in an oxidizing atmosphere to obtain lithium nickelate. The actual reversible capacity of lithium nickelate can reach more than 190mA·h/g.
In general, divalent nickel is more difficult to oxidize to +3 valence, resulting in lithium-deficient lithium nickelate; in addition, if the heat treatment is too high, the generated lithium nickelate is prone to decompose, because the stoichiometric composite oxide is prepared At this time, doping is generally done by adding cobalt. The commonly used solid-phase reaction cannot guarantee the uniformity of its mixing, so the sol-gel method is used to solve this problem. The time required to synthesize the composite oxide LiNiχCo1-χO2 is short and the crystallization performance is good. When χ=0.8, the electrochemical performance is the best, which is better than LiNiO2 or LiCoO2 alone, and the reversible capacity is more than 160mA·h/g. The compound is heat-treated at 700-800°C for 2 to 5 hours, and lithium nickelate with good crystallinity can be obtained.
As mentioned above, in order to prevent lithium nickelate from decomposing due to excessive heat treatment temperature and affecting its electrochemical performance, a sol-gel method using organic matter as a carrier can also be used. The organics used are citric acid and polyvinyl butyral. Taking polyvinyl butyral as an example, lithium nickelate with good crystallinity can be obtained by heat treatment at 750°C for 5 hours. This method is superior to solid phase reaction and spray drying process. One of the reasons is that lithium and nickel are mixed at the atomic level. The other is that organic matter is oxidized during heat treatment, which generates a lot of heat, which can accelerate the formation of lithium nickelate solids. Of course, adding more organic matter is not the better. Too much will cause the partial pressure of oxygen to be low, making the process of nickel oxidation from +2 to +3 incomplete.
Metal manganese is cheaper than metal cobalt and nickel, and the resources are very rich. When the material synthesized by the solid-phase reaction method is used, the valence state of manganese changes between +3 and +4 during the reversible insertion and extraction of lithium, and the size of Mn3+ and Mn4+ are different, resulting in Jahn-Teller The effect is to cause distortion of the spinel microstructure. As a result, the spinel structure of lithium manganate is destroyed as the cycle progresses, and the electrochemical capacity is significantly attenuated.
It is the same as the preparation method of lithium cobaltate and lithium nickelate, that is, the sol-gel method is used to prepare a mixture of manganese salt, lithium hydroxide and ammonia water, and a mixture of nano-scale manganese hydroxide and lithium ions can be prepared. The obtained lithium manganese oxide material still does not fundamentally solve the capacity attenuation problem caused by the Jahn-Teller effect. On the basis of this method, introducing heteroatoms such as nickel, chromium, copper, etc. to modify it can inhibit the Jahn-Teller effect of the material and improve its cycle performance, but the capacity is reduced, generally 100mA·h/g about.
Although the above-mentioned doping method can improve the cycle performance, the material capacity is not high. If organic matter is used as the carrier, it can effectively overcome the shortcomings of low capacity while ensuring its excellent cycle performance. The organics used are small molecules and polymers. Small molecules include tartaric acid, hydroxyglycolic acid, succinic acid, adipic acid, etc., and polymers include polyacrylic acid, condensation polymers of citric acid and ethylene glycol. The cycle performance and capacity of the obtained lithium manganate are significantly improved. The carboxylic acid group of polyacrylic acid forms a complex with the mixed lithium ion and manganese divalent ion, and forms a sol. In this way, the metal ions are uniformly dispersed in the polymer carrier at the molecular level. The resulting gel varies with the ratio of PAA, lithium, and manganese, and can have a cross-linked structure or a non-cross-linked structure. Generally, when PAA is excessive to form a cross-linked structure, segregation will not occur during the heat treatment process. The heat treatment temperature for forming a single spinel structure is also relatively low, which can be as low as 250°C. The reversible capacity of LiMn2O4 obtained at 800℃ is 135mA·h/g (91% of the theoretical capacity). With metallic lithium as the reference electrode, it is 134mA·h/g after 10 cycles, and the attenuation is only 9.5% after 168 cycles. .
When the Li1+χV3O8 cathode material is prepared by the sol-gel method, generally a certain stoichiometric ratio of lithium nitrate or lithium carbonate is used as the lithium source, and ammonium vanadate is used as the vanadium source to dissolve or uniformly disperse in water, and then mix the pre-prepared lemon The acid saturated solution is slowly added dropwise to the lithium vanadium compound mixed solution under stirring. The temperature is raised to 80°C and the reaction is stirred for 0.5h to ensure that the reactants are evenly mixed, so that the lithium ion, the pentavalent vanadium ion and the citrate are fully complexed and reacted. After the reaction is complete, the gel is evaporated and concentrated at about 80°C. The wet gel is formed into a loose foamy Li1+χV3O8 xerogel precursor containing citric acid in the glue at 110~120°C in a vacuum oven; The product was calcined in the air at 450°C for 20 hours. Someone added the stoichiometric ratio of vanadium pentoxide and lithium hydroxide to distilled water, stirred the reaction system for 12 hours, added a small amount of ammonia, and placed the mixture in a water bath at 80°C, evaporating the water, and then vacuum-dried at 120°C 4h, finally calcined at 450°C to obtain the product.
The Li1+χV3O8 cathode material product prepared by the sol-gel method is directly in the form of fine powder, and the product has a uniform particle size, a large specific surface, and a complete structure. It can be directly used as an electrode material without complicated post-processing. It discharges at a constant current at a current density of 0.25mA/cm2, and the actual specific capacity of the first discharge can reach 350mA·h/g. The electrode materials prepared by this method have major breakthroughs in specific capacity and cycle performance.
Due to the high valence of vanadium, it is easily hydrolyzed to form a gel, so there are many types of gels obtained, such as hydrogels, aerogels, and xerogels. The lithium insertion capacity of V2O5 obtained by traditional methods is limited, and generally less than 2 mol lithium per mole of V2O5 unit. If it is prepared into a gel, its lithium insertion capacity is greatly improved, and the amount of lithium insertion per mole of V2O5 unit can be as high as 5.8 mol. The reason for the greatly increased lithium storage capacity may be that the position of lithium insertion has changed on the one hand, resulting in thermodynamics. A better position for lithium insertion; on the other hand, it may be that the interlayer spacing of the material is increased, resulting in a nearly two-dimensional ordered structure of the V2O5 gel, with weak interaction between the layers, so lithium is easier to intercalate.
Generally speaking, the cycle performance of the V2O5 gel prepared by the sol-gel method is not ideal, and the capacity decays relatively quickly. At present, there are two improved methods. One is the same as the preparation of lithium manganese oxide, in which impurities such as iron and aluminum are introduced during the preparation process by the sol-gel method; the other is the sol-gel method. modified. The distribution of the impurity elements introduced by the former is relatively uniform. In the prepared composite oxide, they are connected to the V2O5 belt to increase the interaction between the layers. In this way, the stability of the layered structure is improved during the charging and discharging process, thereby improving Improved cycle performance.
After 40 times, the capacity remained above 410mA·h/g. The latter is the same as an alternative method. Sodium metavanadate is prepared into a hydrogel. Then, instead of directly using the supercritical method for drying, the water in the hydrogel is replaced with acetone, and then cyclohexane is used. The acetone was replaced to prepare an organogel. The organosol cyclohexane in the organogel is removed due to drying during the process of preparing the electrode. Even if a small amount of organic solvent remains, it will not react with lithium and cause irreversible capacity. Its capacity can reach 410mA·h/g, which can reversibly extract about 2.9mol lithium per mole of V2O5 unit, and the capacity decay rate per cycle is as low as 0.19%.
From the above results, the sol-gel method has very obvious advantages in the preparation of lithium-ion battery materials, and it will greatly promote the research and industrialization of lithium-ion batteries. As people's understanding continues to deepen, the sol-gel method will also play a powerful role in promoting the exploration and development of new-type lithium-ion batteries.
For example, adding nano-filler titanium oxide, aluminum oxide, etc. prepared by the sol-gel method to the polyethylene oxide-LiClO4 electrolyte can make the conductivity of the polymer electrolyte reach the application level at room temperature. The improvement of the conductivity of the polymer electrolyte is not like other common methods by adding a liquid plasticizer to the polymer electrolyte to increase the conductivity. Due to the use of solid nano-fillers, there is no liquid plasticizer, which greatly improves the compatibility of metal lithium with the electrode. The specific surface area of the nano-fillers is large, and the mechanical properties of the polymer are not reduced, thus giving birth to all-solid-state lithium batteries. .
In addition, the theoretical research needs to be further deepened. For example, in the lithium manganese oxide prepared by the sol-gel method, why is the Jahn-Teller effect not obvious? Logically, there is also a conversion between Mn3+ and Mn4+. One of the reasons may be the same as the preparation of lithium nickel oxide, the ordering process of Li+ has been improved. If this problem can be completely solved, the cost of lithium-ion batteries will be greatly reduced.