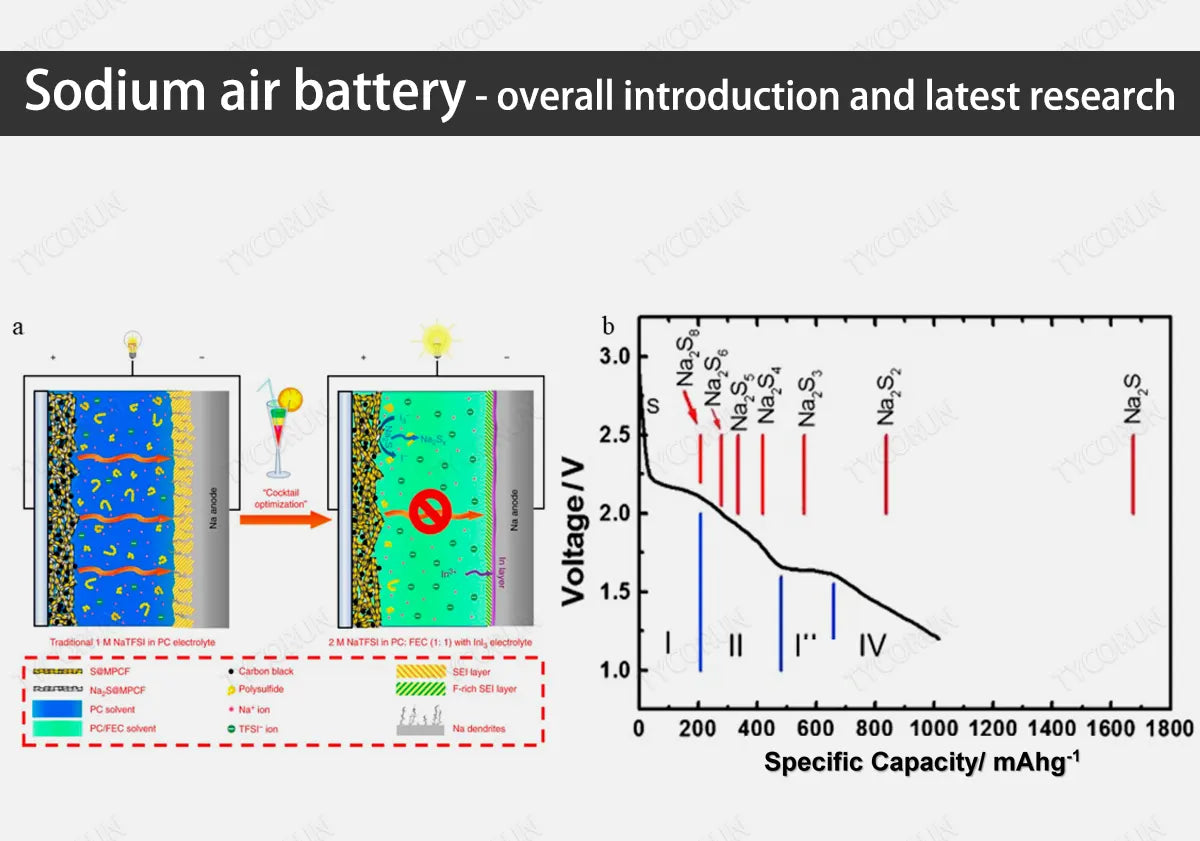
With the strengthening of global environmental awareness, the development of environmentally sustainable and high-energy-density energy has gradually become the focus of attention. In recent years, metal-air batteries have attracted widespread attention as energy storage devices due to their high energy density, and sodium air battery is also one of them.
Main content:
The rapid development of portable and flexible electric devices requires that their energy supply systems not only have high energy density, but also have high flexibility to adapt to the complex application scenarios faced by flexible electric devices.
Metal sodium air battery has a high energy density of up to 1108 watt-hours per kilogram and are low-cost, so they are considered to be widely used in future flexible electric devices. The most important thing is that the reactant of this type of battery is oxygen in the air and does not require auxiliary equipment to store it, making it superior to other secondary batteries in terms of quality and volume.
Considering the shortage of metallic lithium resources and the similar physical and chemical properties of metallic sodium and lithium, replacing metallic lithium with metallic sodium has become a hot topic in battery research. Sodium air battery has also attracted the interest of researchers and sodium ion battery companies as a future energy storage device.
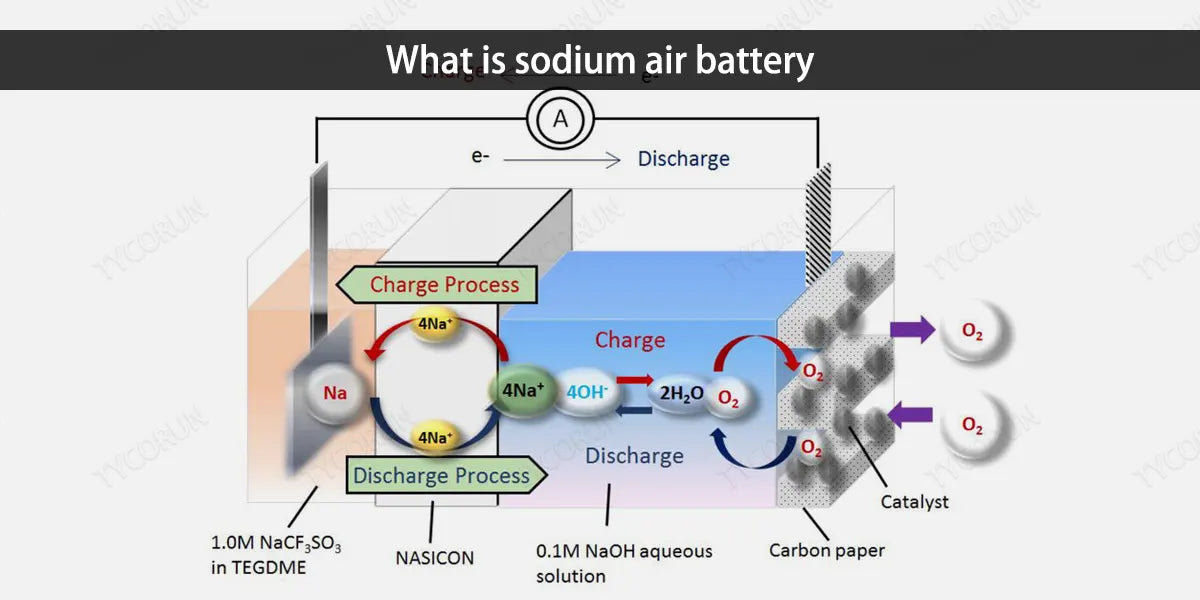
1. What is sodium air battery
Metal-air batteries are secondary batteries that use metal as the negative electrode for oxidation reaction, and air or oxygen for reduction reaction at the cathode to achieve current output. Sodium air batteries belong to a metal-air battery system.
Alkali metal ions and oxygen react on the air electrode to produce alkali metal oxides to drive the entire battery. The positive electrode usually uses porous carbon materials or porous metal materials, which not only provide channels for the transmission of oxygen, but also provide reaction sites for the reduction of oxygen and the combination with alkali metal ions to form alkali gold oxides.
The alkali metal oxides generated during the discharge process will continue to fill the gaps in these porous materials, and the discharge reaction will not terminate until the gaps are completely filled.
Sodium air battery is one of the high-energy battery technologies that is expected to be used as a power battery in the future. It is also considered to be one of the most promising secondary batteries to replace lfp battery.
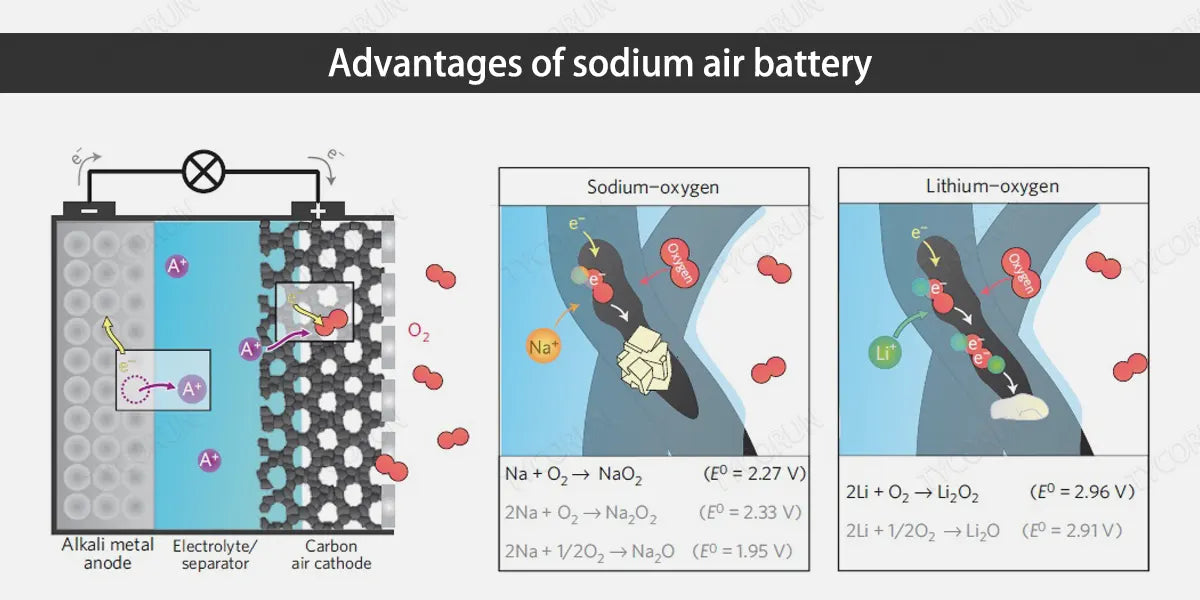
2. Advantages of sodium air battery
- High energy density: According to gasoline standards, for transportation and grid energy storage, commercial battery energy density requirements are about 1700Wh/kg, and sodium air batteries can reach 1600Wh/kg, which has a higher energy density.
- High energy efficiency: low charging voltage greatly improves the energy efficiency of the battery. Organic electrolyte is usually used as the electrolyte in non-aqueous sodium air battery. The lower charging voltage can avoid the problem of decomposition of organic electrolyte due to the narrow electrochemical window and reduce the occurrence of side reactions.
- Price advantage: The abundant resources and low price make sodium air batteries suitable for large-scale production and application and work with 3000w inverter.
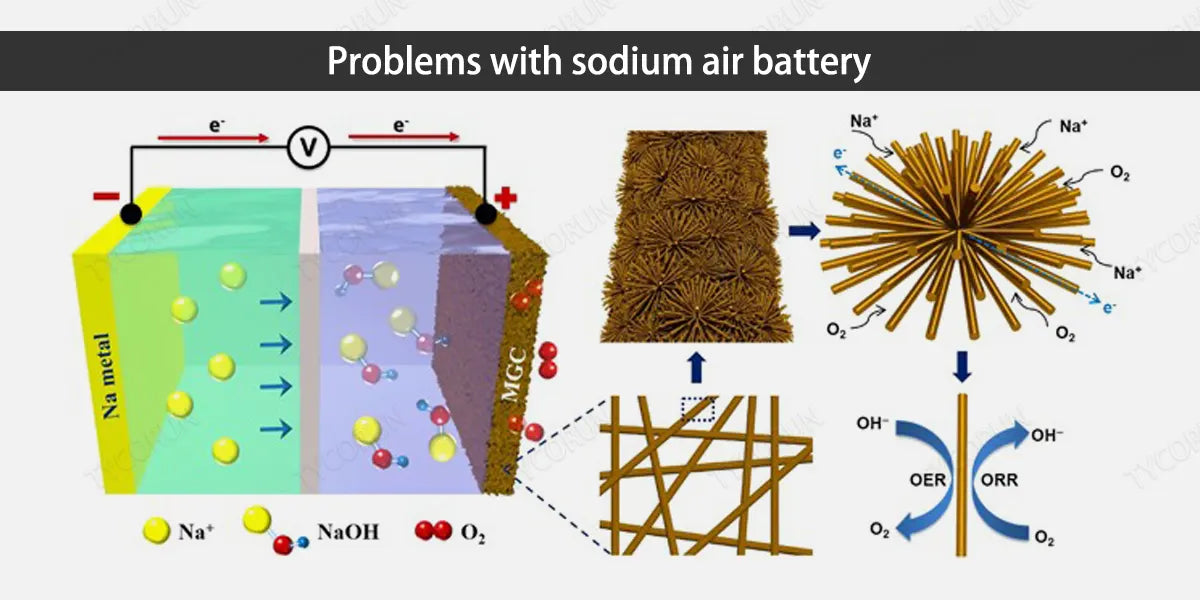
3. Problems with sodium air battery
- The reaction mechanism of non-aqueous sodium air battery is still unclear and controversial.
- Non-aqueous sodium air battery mostly uses organic solutions as electrolytes. Organic electrolytes are unstable and have problems of combustion and leakage, and have poor safety performance. The discharge product sodium peroxide or sodium superoxide, which is insoluble in the organic electrolyte, causing gas channel blockage and affect battery life.
- Aqueous (mixed) sodium air battery mostly uses sodium hydroxide solution as the electrolyte. The sodium hydroxide electrolyte volatilizes seriously and the battery life is short. The occurrence of side reactions such as hydrogen evolution reaction needs further confirmation.
- Sodium dendrites exist in both aqueous and non-aqueous (mixed) sodium air battery. The growth of sodium dendrites will pierce the separator, causing the battery to short circuit or even explode. There is currently no proper solution for this problem.
- The working conditions of aqueous and non-aqueous (mixed) sodium air battery are usually restricted. Sodium is very sensitive to moisture. Contact between moisture and anode metal sodium will cause a short circuit in the battery, and entering the catholyte will cause the electrolyte concentration to change. Carbon dioxide easily reacts with discharge products to form an insoluble product - sodium carbonate. Sodium carbonate is non-conductive and has poor reversibility, which will block the gas channels.
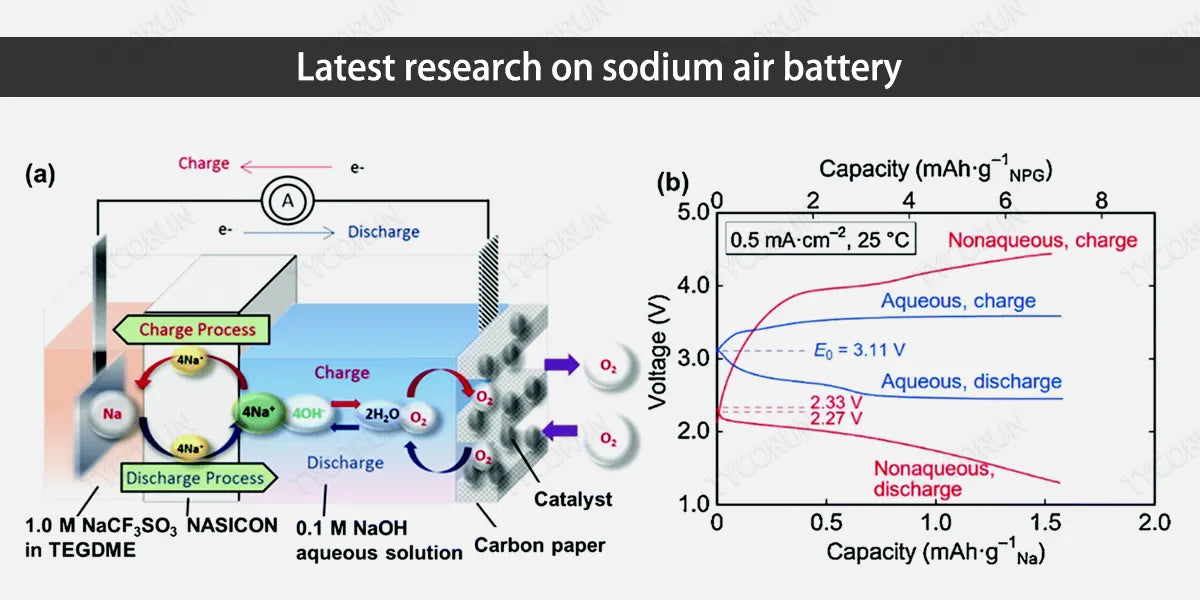
4. Latest research on sodium air battery
As mentioned above, sodium air battery is currently facing many problems such as low catalytic activity in oxygen reduction/evolution reactions and the formation of sodium dendrites in the negative electrode, which affect the practical application of batteries. An in-depth discussion of these issues relies on a deeper understanding of battery charging and discharging mechanisms and interface issues.
Currently, research on sodium air battery mainly focuses on the design and preparation of new cathodes and electrolytes to improve the electrochemical performance of traditional rigid sodium air battery. However, for flexible sodium air battery, metallic sodium anode is the key restricting its practical application. Metal sodium is soft in texture.
Under deformation, on the one hand, a large number of defects and cracks will occur on the surface of the sodium metal anode, which will intensify the growth of sodium dendrites, leading to serious consequences such as battery short circuit and even fire and explosion.
On the other hand, deformation will aggravate the pulverization of the metallic sodium negative electrode, causing the battery performance to decline rapidly. In recent years, many works have been reported on suppressing dendrites in metallic sodium anodes, such as designing electrolytes, constructing artificial solid-electrolyte interface films, and structured metal anodes.
Although these strategies have a good dendrite suppression effect in traditional planar metal anodes, they have not paid attention to the growth behavior of metallic sodium under dynamic deformation.
In this regard, some researchers designed and prepared a protective layer of sodium carbon nanotubes to homogenize the surface potential and current distribution of metallic sodium, achieving a flexible metallic sodium anode that can work stably under dynamic deformation conditions.
Furthermore, based on this stable flexible metal sodium anode, a fiber sodium-air battery with high flexibility and high electrochemical performance was constructed, which provides new research ideas for the practical application of flexible metal-air batteries, including 12v 200ah deep cycle battery.
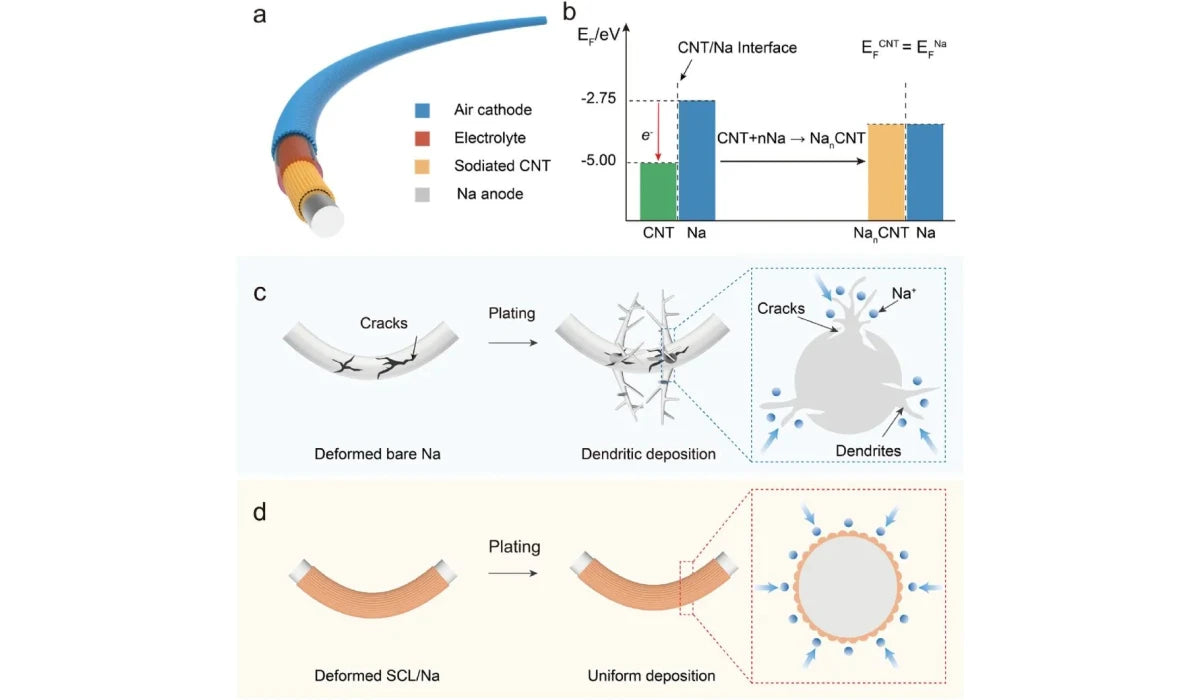
a. Structure of fiber sodium air battery
b. Formation mechanism of the protective layer of sodium carbon nanotubes
c. The process of dendrite formation in bare metal sodium anode under deformation conditions
d. The stabilizing effect of the sodium carbon nanotube layer on the deposition process of metallic sodium anode
Through a simple and easy in-situ synthesis method, a new type of sodium carbon nanotube protective layer can be prepared on the surface of fiber metal sodium anode. The carbon nanotube film soaked in the electrolyte is evenly coated on the surface of the metallic sodium negative electrode.
Due to the different Fermi energy levels of the carbon nanotubes and the metallic sodium, the contact potential difference generated between the two can cause the electrolyte to decompose and form a stable solid-electrolyte interface film on the surface of the carbon nanotube.
Theoretical simulation results show that after deformation, there is a locally enhanced potential at the defect site on the surface of the bare metal sodium ion battery anode, which attracts sodium ions to concentrate and deposit at the defect site to form dendrites (Figure a, b).
In contrast, in the sodium carbon nanotube layer/metal sodium composite electrode, the surface potential and current density are evenly distributed, allowing the metal sodium to be uniformly deposited and avoiding the formation of dendrites (Figure c, d).
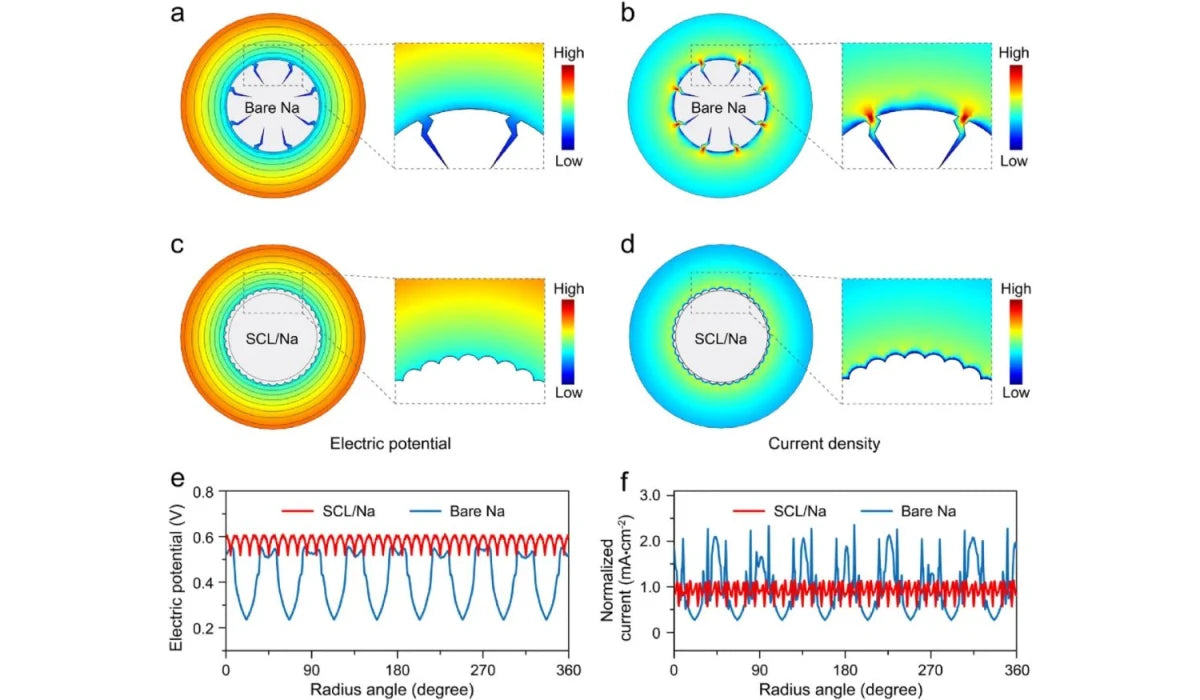
(a,b) Surface potential and current density distribution after deformation of bare metal sodium anode
(c,d) Surface potential and current density distribution after deformation of the sodium carbon nanotube layer/metal sodium composite electrode
(e) Surface potential distribution curves in bare metal sodium negative electrode and sodium carbon nanotube layer/metal sodium composite electrode
(f) Surface current density distribution curves in bare metal sodium anode and sodium carbon nanotube layer/metal sodium composite electrode
Flexible sodium air battery needs to face huge deformations during actual use, such as bending, twisting, etc., which aggravate the dendrite growth problem of metal anodes. The above-mentioned sodium carbon nanotube protective layer design can homogenize the surface potential and current distribution of metallic sodium, achieving a flexible metallic sodium anode that can work stably under dynamic deformation conditions.
Furthermore, based on this stable flexible metallic sodium anode, a fiber sodium-air battery with high flexibility and high electrochemical performance was constructed, providing some new ideas for the development of new flexible high-capacity energy storage systems.
Related posts: Lithium vs sodium battery, Sodium sulfur battery, 18650 rechargeable battery