
Main content:
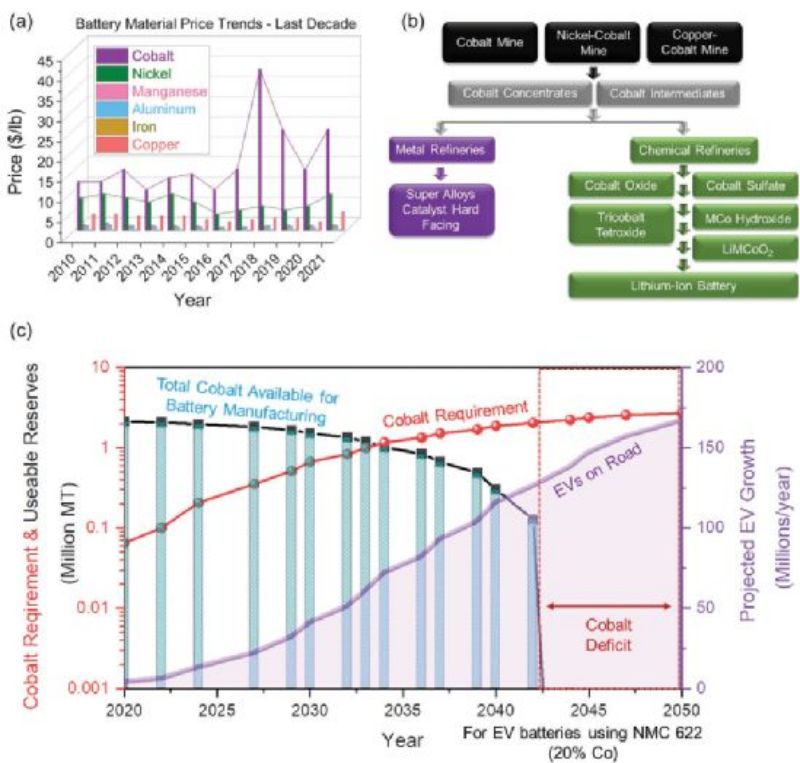
Hundreds of millions of electric vehicles (EVs) are expected to be on the road by 2050, and this growing demand threatens to deplete global cobalt reserves at an alarming rate. In addition, the tightening of the cobalt supply chain has driven up cobalt prices significantly over the past decade. Therefore, the dependence on cobalt needs to be reduced to meet the increasing demand for lithium-ion batteries. In view of this, Nitin Muralidharan and Ilias Belharouak et al. from The Us Treeridge National Laboratory published a review paper in Advanced Energy Materials summarizing recent advances in cobalt free lithium ion batteries cathode research. They include layered, spinel, olivine and disordered halite type materials. Despite the good performance of these cobalt-free cathode electrodes, the problem of large-scale production still needs to be solved.
Cobalt prices have nearly tripled in recent years as supply chain lock-ups have led to increased demand (Figure 1A), creating an unpredictable situation for battery manufacturing. Cobalt is now nearly 60 per cent more expensive than nickel, the second key element in LIBs. According to a recent report by the Cobal Development Institute, about 40% of the world's cobalt is used in lithium-ion batteries, with the remaining 60% in other uses, including catalysts, magnets, superalloys and pigments (Figure 1B). The statistics highlight how limited cobalt supplies could hamper the growth of the electric vehicle market. Global demand for cobalt will exceed reserves by 2045 unless cobalt free lithium ion batteries cathode electrodes or recycling solutions are adopted for electric vehicle batteries (Figure 1C)
1. Market potential of cobalt free lithium ion batteries
As shown in Figure 2, if LiNi0.8CO0.15Al0.05O2 (NCA) cathode electrode is used, producing 1 million electric vehicles requires nearly 10 KTS of cobalt (Figure 2). Similarly, production of more than 150 million electric vehicles using the NMC622 cathode by 2050 would require more than 2.5 million tons of available cobalt, representing more than one third of known global cobalt reserves (≈7 million tons). Therefore, the high cobalt cathode electrode cannot meet the demand of future electric vehicles, and it is unrealistic to expect battery manufacturers to offset the high cost of cobalt in a limited supply chain market. Transitioning to cobalt-free materials is the most straightforward way to meet the demand for affordable electric vehicles in the decades ahead. The analysis in Figure 2 estimates that the adoption of a cobalt free lithium ion batteries cathode electrode will significantly reduce lithium ion battery prices and costs ($8,500 for total packaging and $3,500 for materials) compared to a battery system containing an NCA cathode electrode ($10,000 for total packaging and $5,000 for materials).
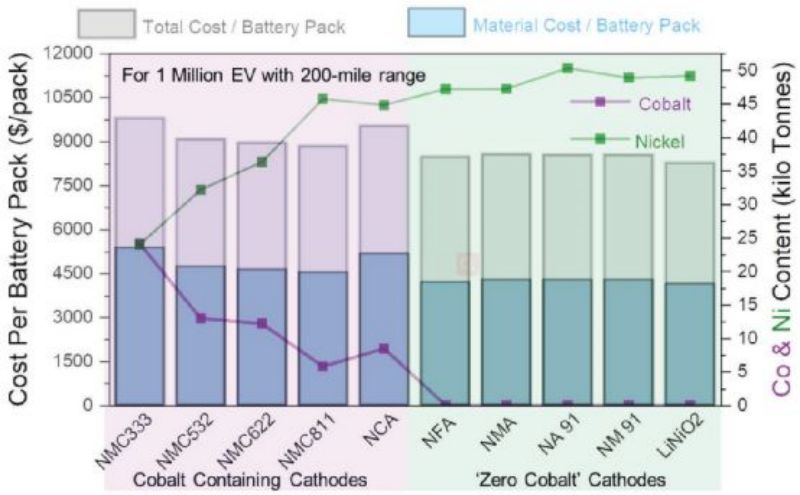
FIG. 2
2. Layered cobalt oxide cathode electrode
The cathode pole selection has the greatest impact on LIB cost and performance. To make low-cobalt/cobalt free lithium ion batteries widely available for EV applications, a full understanding of the challenges associated with cathode performance and manufacturing is required. The following sections discuss these issues for several promising low-cobalt/cobalt-free cathode, including lamellar, spinel, olivine, and DRX, as shown in Figure 3.
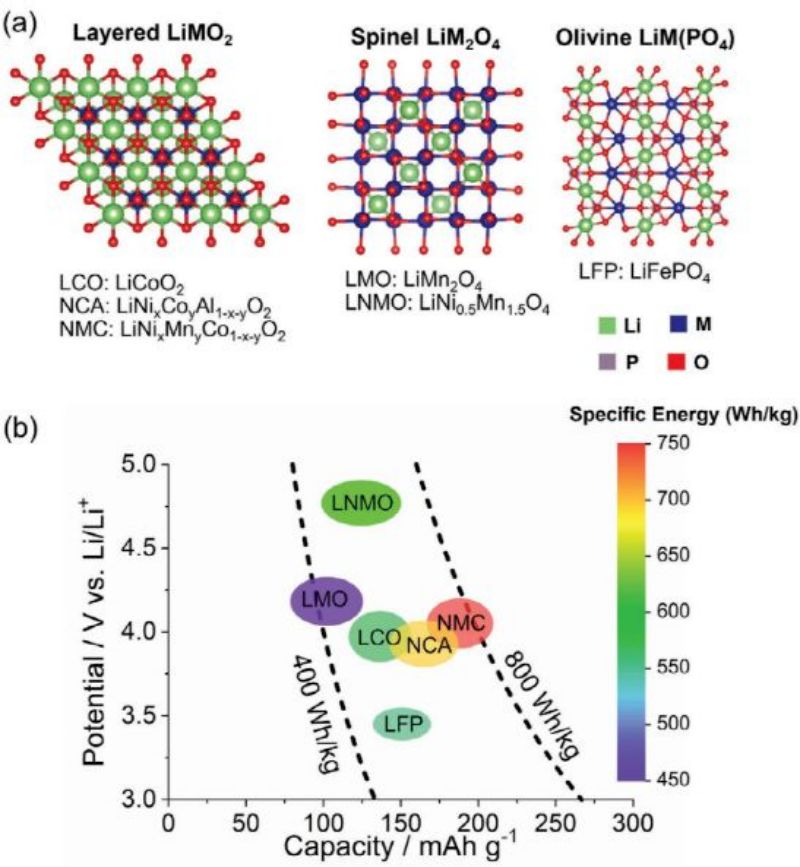
FIG. 3
FIG. 4A and B show the constant-current charge-discharge curve and cycle performance of LNO without cobalt and manganese. Increasing Mn content improves cyclic stability, but at the expense of discharge capacity (e.g., 212 mAh g−1 (10% Manganese) for 164 mAh g−1 (50%Mn)), due to the low electrochemical activity of Mn4+ ions. FIG. 4C and D show the thermal stability of manganese doped LNO electrode. The cathode electrode with low manganese content produced relatively less heat in the thermal decomposition test (901.4 J g−1 for 10% manganese and 485.7 J g−1 for 50% manganese). It is speculated that the spinel phase formation improves the structural integrity of the 10% Mn doped LNO sample and delays the onset of exothermic reactions.
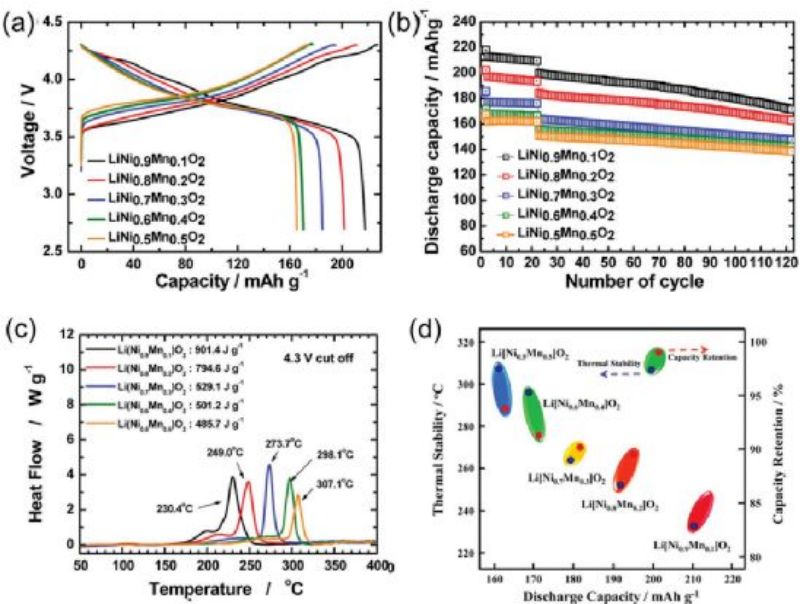
FIG. 4
Mg and Al doping has been widely reported to improve the electrochemical performance of cathode LNO electrodes. It has been shown that Mg doping can improve cyclic stability by reducing the cracking of cathode particles during lithium insertion/removal. Li et Al conducted a systematic study on the doping of Al (Lini0.9Al0.1O2), Mg (Lini0.9Mg0.1O2), Co (LinI0.95CO0.05O2) and Mn (Lini0.9Mn0.1O2) (FIG. 5A-c). Based on the differential capacitance diagram shown in FIG. 5a, the authors suggest that by adding Al, Mn, and Mg ions to the transition metal layer, the cathode electrode of LNO inhibits the harmful phase transition during delithium/lithium.
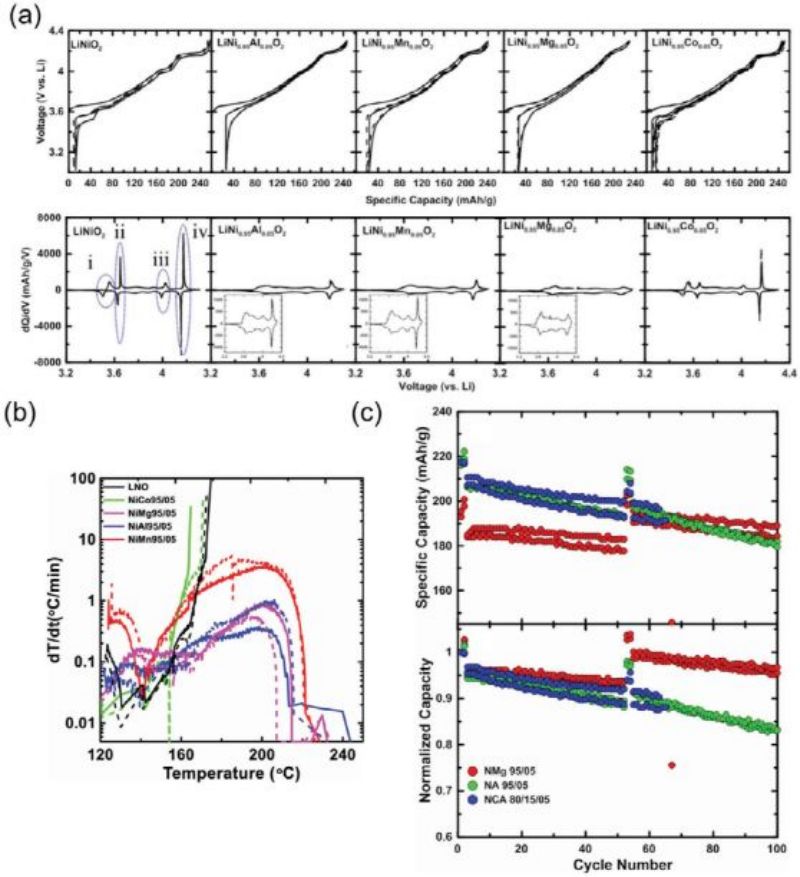
FIG. 5
FIG. 6A shows a schematic of the benefits of combining iron and aluminum and the resulting crystal structure. The neutron diffraction pattern of the NFA cathode component variant shows a rhomboid crystal structure with image space point groups corresponding to the α -Nafeo2 crystal structure, as shown in FIG. 6b. As mentioned above, due to the similar ionic radii of Ni2+ and Li+, the structure of nickel-rich cathode electrodes may be affected by cation mixing. Using Rietveld refinement and neutron diffraction analysis, we determined that the formation of antidislocation defects accounted for about 4% of the cathode NFA variants.
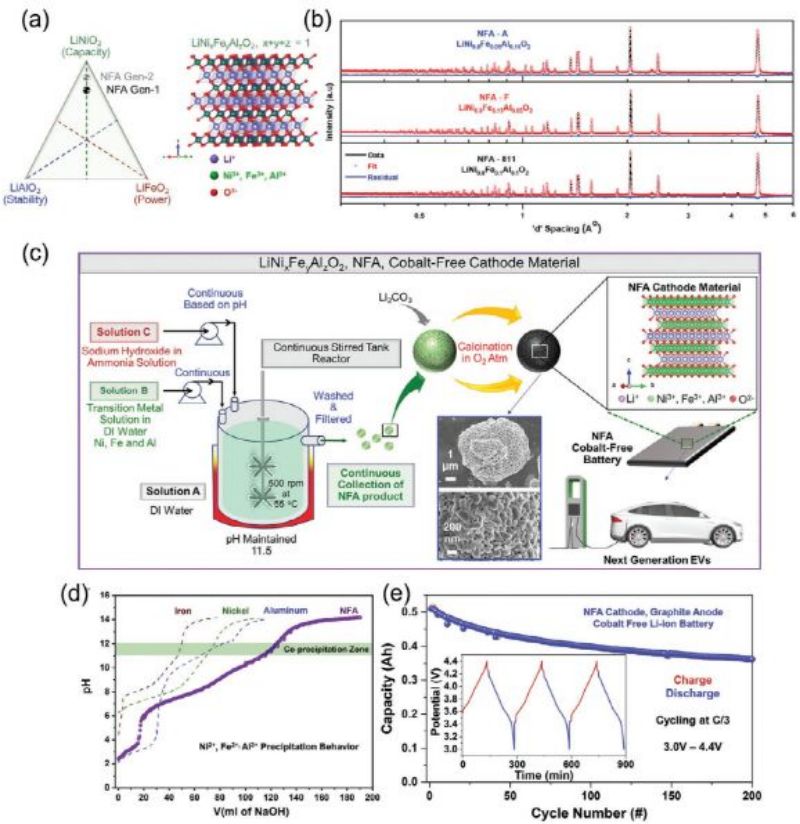
FIG. 6
In recent years, several other types of cobalt free lithium ion batteries ternary cathodes have been reported, including LiNixMnyGzO2 (NMM), LiNixNyTizO2 (NMT), LiNixNyAlzO2 (NMA) (x+y+z=1, x>60%). All of these cathode electrodes have a capacity greater than 200 mAh g−1 when charged to 4.5V (vs. Li/Li+) (FIG. 7a, b). Electrochemical cycling performance tests showed (FIG. 7c) that these materials provided a higher capacity after 100 cycles, with a capacity retention rate of >80%.
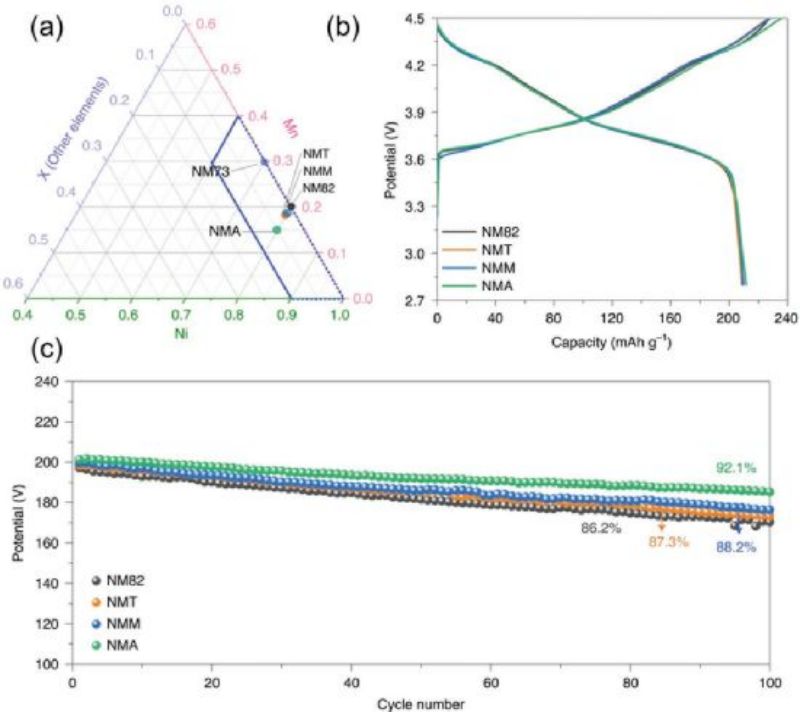
3. Spinel and olivine anode
The schematic diagram of spinel structure is shown in Figure 3A. Thackeray et al. first reported LiMn2O4 spinel cathode material (0<x<0.8 in="" li1−xmn2o4).
4. DRX cathode material
The cathode DRX electrode contains randomly arranged lithium ions and transition metal (TM) ions, as shown in Figure 8a (α -LifeO2 structure). Due to the wider distribution of the local bonding environment, the cathode DRX electrode has a unique Li+ diffusion path.
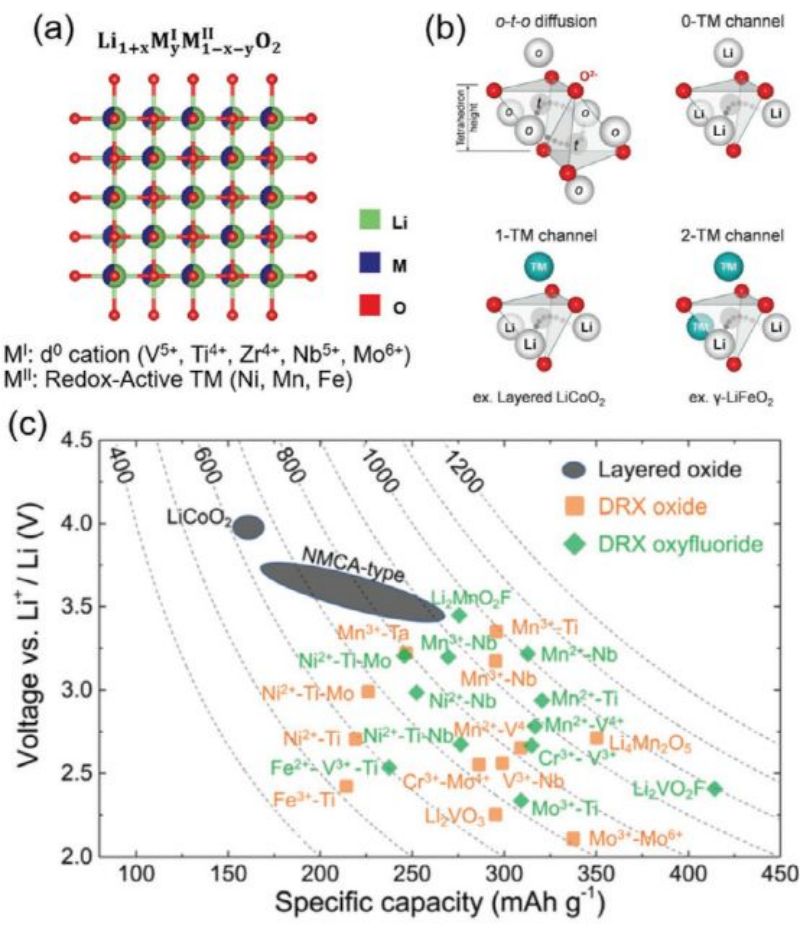
FOG. 8
5. Challenges of new cobalt free lithium ion batteries in commercial applications
While cobalt-free cathodes are expected to be used in the next generation of lithium-ion batteries, these new materials should be seamlessly integrated into the existing manufacturing infrastructure. Industrial manufacturing of lithium-ion batteries relies on two key industries, both of which have their own unique challenges: I) cathode synthesis and ii) electrode processing and battery assembly. The co-precipitation routes of intermittent and CSTR reactors are widely used in the manufacturing of cathode electrode precursor systems in modern commercial scale manufacturing processes. The precursor is then converted to the final lithium form through further processing, including mixing and calcination. Therefore, the commercialization of cobalt-free cathode is faced with the challenges of precursor synthesis, final processing and molding of cathode powder, electrode manufacturing, and battery assembly.

6. Summary and Prospect
This paper reviews the application of low cobalt/cobalt free cathode in high energy and low cost lithium ion batteries. Although cobalt-free cathode poles such as LiMn2O4 (spinel) and LiFePO4 (olivine) have been commercialized in some applications, the energy density of these materials is still low compared to LiCoO2. In addition, commercial nickel-rich layered oxides, such as NMC and NCA, show good performance. Recent reports of cobalt-free cathode electrodes typically conduct charge/discharge cycles at a rate of at least C/3, which is relevant for the practical application of electric vehicles. It is expected that further optimization of cobalt free lithium ion batteries materials will yield better rate performance. In addition, as energy storage demand continues to grow in the coming decades, over-reliance on nickel-based cathodes could become a problem. The cathode DRX electrode with high cationic disorder is another potential choice for the next generation of lithium.
Literature information:
Muralidharan, N., Self, E. C., Dixit, M., Du, Z., Essehli, R., Amin, R., Nanda, J., Belharouak, I., Next-Generation Cobalt-Free Cathodes – A Prospective Solution to the Battery Industry's Cobalt Problem. Adv. Energy Mater. 2022, 12, 2103050.