There is no clear boundary between crystalline and amorphous materials, and they can be transformed into each other under certain conditions.For carbon materials, this phenomenon is even more prominent. They all contain graphite crystals and amorphous regions, but their relative content is different. Amorphous carbon materials, they are also composed of graphite crystallites, and the carbon atoms are combined in sp² hybrid mode, but their crystallinity is low, La and Lc values are small, and the structure of graphite flakes is not as regular as graphite. Ordered, so it does not show the nature of crystals macroscopically.

amorphous carbon
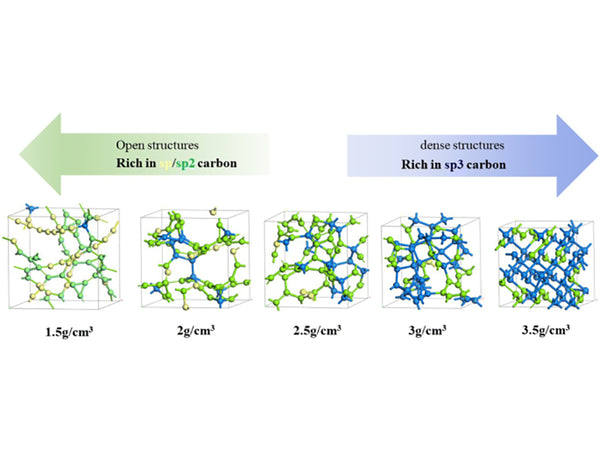
Amorphous carbon materials can be divided into easy graphitization carbon and difficult graphitization carbon according to their degree of graphitization difficulty. Easily graphitized carbon, also known as soft carbon, refers to amorphous carbon that can be graphitized at a high temperature above 2500°C; non-graphitizable carbon is also called hard carbon, which is also difficult to graphitize at a high temperature above 2500°C. The reason why amorphous carbon materials are divided into soft carbon and hard carbon is mainly due to the different arrangement of the graphite flakes that compose them. Figure 1 is a structural model of soft carbon and hard carbon.

Figure 1 Structure model of soft carbon and hard carbon
All carbon materials are made up of similar basic structural units (also called graphite crystallites) that are cross-linked and arranged in different ways. The basic structural unit is composed of 2~4 layers of carbon hexagonal nets composed of 10-20 aromatic rings, which overlap in a more or less parallel manner. When the precursor is decomposed in the soft carbon, colloids are formed to make the carbon basic structural unit long The macromolecules of the organic precursor of hard carbon are fully cross-linked, and colloids are not formed, and the basic structural units cannot be arranged in parallel. Graphitization is difficult at any temperature.
Generally speaking, the raw material undergoes solid-phase carbonization to obtain non-graphitizable carbon; the raw material undergoes liquid phase carbonization to obtain easily graphitized carbon. In the liquid phase carbonization process, the more complete the mesophase thermal conversion process proceeds, the easier graphitization of the obtained carbon material is. . The changes of the structural parameter d002 of the two types of carbon during high temperature heat treatment are shown in Figure 2.
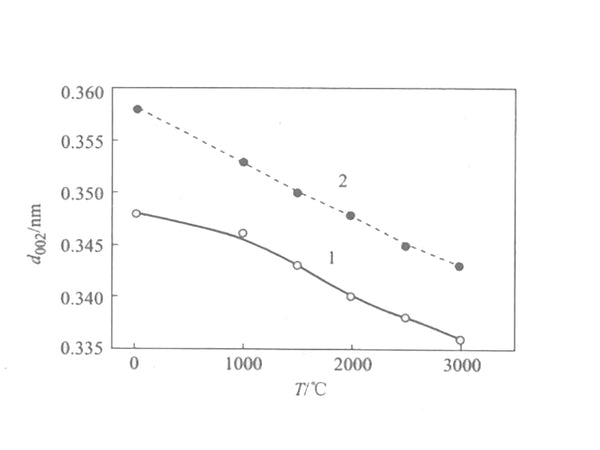
Figure 2 The change curve of the structure parameter d002 of amorphous carbon with temperature
1—soft charcoal; 2—hard charcoal
It can be seen from the figure that the interlayer spacing d002 of soft carbon is smaller than that of hard carbon, and with the increase of temperature, it is closer to the interlayer spacing d002 of graphite (0.3354nm); the layer thickness Lc of soft carbon and hard carbon is relatively small at low temperature. Only a few to a dozen nm, after high temperature treatment, the Lc of soft carbon changes significantly, while the change of Lc of hard carbon is not very obvious.
No matter what degree of graphitization can be achieved, amorphous carbon materials treated at 500~1000°C have the following common characteristics. First, the degree of crystallinity is low, and the XRD spectrum has some weaker broad peaks. The most common peaks appear near the 002, 100, and 110 crystal planes of graphite. The minimum value of d002 is 0.344nm, in some cases it can reach 0.4nm. Second, it contains tar-like unorganized carbon. They are usually single-layer carbon hexagonal mesh planes or sp3 carbons of varying sizes, which are filled between graphite crystallites or exist in the form of branches and bridges. Third, it has a large number of nanopores, which can be observed with an electron microscope or SAXS (small angle X-ray scattering). At the same time, the density of these carbons is 1.4~2.0g/cm3, which is smaller than graphite, which also reflects that it is porous. sex. Fourth, there are many heteroatoms, which are converted from precursors. There are O, N, H, and S. Their content is related to the precursor and the processing method. Most of the hydrogen can be removed at 1000°C, and O and N are about 1500°C. Loss, and S is not lower than 2000 ℃. They are combined with graphite crystallites or carbon in the carbon hexagonal net plane in the form of functional groups, or combined with the carbon hexagonal net plane in the form of atoms.
Most amorphous carbon materials have high specific capacity, but the irreversible capacity is also high, the first charge and discharge efficiency is low, and the cycle performance is not ideal; the capacity of amorphous carbon materials is related to the heat treatment temperature. As shown in Figure 3, the capacity of most soft carbons and some hard carbons first showed a downward trend with the increase of heat treatment temperature. The capacity of soft carbons did not rise again until around 1900°C, while the capacity of hard carbons increased slightly after 2000°C. The lithium storage mechanism of amorphous carbon materials at low temperature is more complicated, and it is related to the degree of graphitization at high temperature.
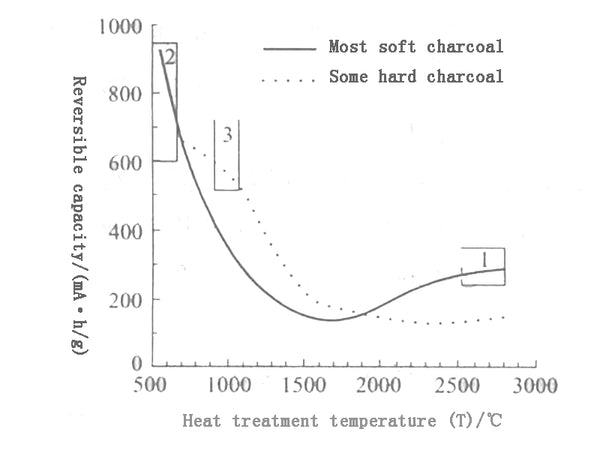
Figure 3 The relationship between heat treatment temperature and reversible capacity of different carbon materials
Amorphous carbon materials processed at about 700°C have voltage hysteresis: the insertion of lithium is about 0V, but the extraction of lithium is about 1V; and the difficult-graphitizable carbon materials processed at 1000°C have a lower voltage plateau and a voltage hysteresis. It is small, but has a steep slope on its voltage distribution curve.
1.Easily graphitized carbon
Easily graphitized carbon mainly includes coke, carbon fiber, non-graphitizing mesophase carbon microspheres, etc. The most common coke-based material used in lithium-ion batteries is petroleum coke because of its abundant resources and low price; carbon fiber mainly refers to vapor-grown carbon fiber and mesophase pitch-based carbon fiber.
Coke is a type of amorphous carbon material formed by liquid phase carbonization. It is easy to graphitize at high temperature and is a soft carbon material. Depending on the different raw materials, coke can be divided into pitch coke, petroleum coke, etc. Coke can be regarded as carbon with an underdeveloped graphite structure in nature. The carbon layers are roughly arranged in parallel, but the mesh surface is small and the build-up is irregular. It is a disordered layer structure. The layer spacing d002 is 0.334~0.335nm, which is significantly larger than ideal graphite. The layer spacing.
Petroleum coke is a kind of coke, which is produced by deoxidizing and dehydrogenating petroleum pitch at about 1000°C. The first-generation lithium-ion secondary battery launched by Sony's F in 1990 uses petroleum coke as the negative electrode material. According to the structure and appearance of petroleum coke, petroleum coke products can be divided into four types: needle coke, sponge coke, shot coke and powder coke. Among them, the shape of needle coke particles is slender, needle-like streaks are obvious, and the content of fiber-type microscopic components is high. , The graphitization performance is the best.
Petroleum coke has an amorphous structure, is in the shape of a turbine layer, and contains a certain amount of impurities. It is difficult to prepare high-purity carbon but is rich in resources and low in price. The maximum theoretical chemical lithium insertion capacity of petroleum coke is LiC12, and the electrochemical specific capacity is 186mA•h/g, but the performance of coke itself as a battery negative electrode material is very poor, mainly due to the volume expansion of carbon materials when lithium is inserted. Reduce battery life. Therefore, the coke must be properly modified to increase the charge and discharge capacity of the coke and improve its performance. For example, the reversible capacity of petroleum coke can be increased from 170mA·h/g to 300mA·h/g through the coating of mesophase carbon. Figure 4 shows the charge-discharge curve of coke.
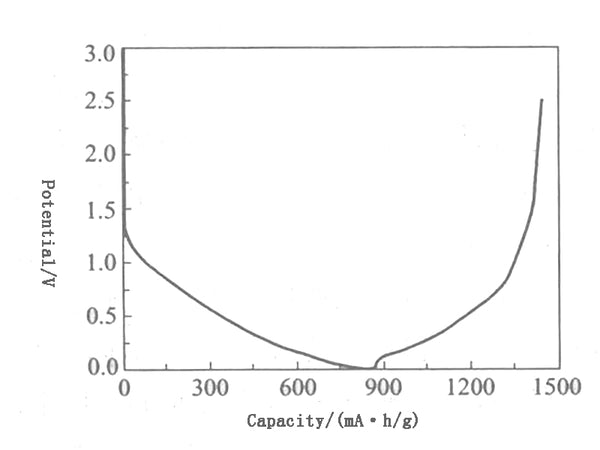
Figure 4 Charging and discharging curve of coke
Petroleum coke is highly adaptable to various electrolytes, and has better resistance to overcharge and overdischarge. However, unlike graphite, there is no platform on the charge and discharge potential curve. It shows a slope in the range of 0~1.2V, and the average The potential for lithium is higher, about 1V, resulting in lower battery terminal voltage, which limits the battery's capacity and energy density.
2. Difficult to graphitize carbon
The non-graphitizable carbon is a pyrolysis carbon of high molecular polymer, which is formed by direct carbonization of the solid phase. In the early stage of carbonization, three-dimensional crosslinks are formed by sp3 hybridization, which prevents the parallel growth of the mesh surface, so it has an amorphous structure and is difficult to graphitize even at high temperatures. The reason why carbon materials such as hard-graphitizable carbon have attracted widespread attention should first be the successful use of poly furfryl alcohol (PFA) carbon by Sony. There are many types of polymer precursors used as difficult graphitized carbon, including some resins and other polymers, such as phenolic resin, epoxy resin, melamine resin, polyfurfuryl alcohol (PFA), Polyphenylene (PP), polyacrylonitrile (PAN), polyvinyl chloride (PVC), polyvinylidene fluoride (PVDF), polyphenylene sulfide, polynaphthalene, cellulose, etc. In addition, carbon black (acetylene black AB) and benzene carbon (BC) are also difficult to graphitize carbon materials.
The charge-discharge curve of a typical hard-graphitizable carbon material is shown in Figure 5. It is different from the charge-discharge curve of graphite and easily graphitized carbon materials, and has a larger first irreversible capacity (generally greater than 20%) and voltage hysteresis. (The lithium removal potential is significantly higher than the lithium insertion potential), the lithium removal potential is high, and the potential platform is not obvious. In addition, its d002 is also large, the solid phase diffuses faster, which is helpful for rapid charge and discharge; it is also compatible with PC (propylene carbonate). In addition to the formation of the SEI film, the non-graphitizable carbon has a large irreversible capacity. The active groups on the surface of the material, such as hydroxyl groups, as well as the adsorbed water are also important reasons.
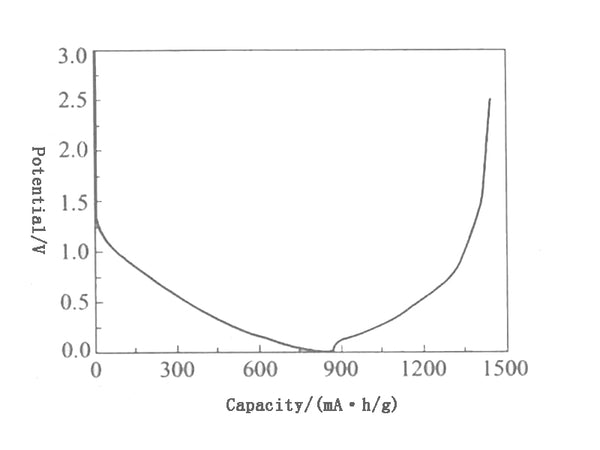
Figure 5 Charging and discharging curves of hard-to-graphitize carbon materials
The amorphous polyethine semiconductor (PAS) obtained by pyrolysis of phenolic resin below 800°C has a capacity of 800mA·h/g and a crystal interplanar spacing of 0.37 ~ 0.40nm, which is equivalent to that of LiC6. This facilitates the insertion of lithium in it without causing significant structural expansion, and has good cycle performance. In addition, PAS reacts with electrolyte and generates low heat. With PAS as the negative electrode, the LiCoO2 18650 commercial lithium-ion battery has a volume specific energy of 450W·h/L, almost reaching the volume specific energy of lithium metal.
The pyrolysis carbon product of polyparaphenylene (PPP) at 700℃ is used as the negative electrode, and the reversible lithium intercalation capacity is as high as 680mA·h/g. Studies have shown that there is no obvious crystal structure in the PPP-based pyrolytic carbon material, only the carbon layer with defects, the spacing between the plane layers is 0.40nm, and the fluctuation is large.
Under suitable pyrolysis conditions, starch, oak, peach core shell, almond shell, maple, lignin, etc. can be used as precursors to obtain pyrolytic charcoal with a reversible capacity of 400~600mA·h/g. Organic polymers such as polyacrylonitrile, poly-4-vinylpyridine, melamine resin, and urea-formaldehyde resin are used as precursors. When the heat treatment temperature is lower than 700℃, as the degree of crosslinking of the polymer precursor increases, the The reversible capacity gradually increases and exceeds the theoretical capacity of graphite.
The structure of non-graphitizable carbon materials contains a certain amount of hydrogen, and the amount of hydrogen content is related to the heat treatment temperature. The carbon obtained by pyrolyzing high polymers at about 1000°C does not contain or contains trace amounts of hydrogen (mH/mC<0.05), and heat at 800°C The proportion of hydrogen in the carbon obtained by decomposing high polymer is relatively high.