
Main content:
Zinc air battery (non-rechargeable) and zinc-air fuel cells (rechargeable) are structurally specific varieties. The anode (negative electrode) uses a zinc alloy. The cathode (positive electrode) material is oxygen in the air. It is generally kept sealed in storage, so there is basically no self-discharge, but it will release continuously once it is used.
Zinc air battery, also known as zinc-oxygen batteries, are sometimes called zinc air battery. Because the positive electrode (cathode) uses air directly, the energy density is relatively high, and its size ranges from button batteries in hearing aids, to large batteries in movie cameras that used mercury batteries, to very large batteries in electric vehicles provide power.
Charging process of zinc air battery
The charging process of zinc air battery is very slow. To solve this problem, usually the negative electrode zinc plate or zinc particles of zinc air battery are oxidized to zinc oxide and fail, and the zinc plate or zinc particles and electrolyte are generally replaced directly. So the zinc air battery can be completely updated.Zinc air battery structure
Zinc air battery has zinc powder in paste form on the anode side and catalytic carbon on the cathode side. Holes in the battery casing allow oxygen from the air to enter the cavity and attach to the carbon in the cathode. At the same time, the zinc at the anode is oxidized, a chemical reaction similar to that of small silver-oxygen or mercury-oxygen batteries.
Cathode - is the catalytic carbon that absorbs oxygen from the air.
Anode - is a mixture of zinc powder and electrolyte, into a paste.
Electrolyte - high concentration potassium hydroxide aqueous solution.
Isolation layer - used to isolate the movement of solid particles between the two stages.
Insulating and Sealing Gaskets - Nylon material.
Battery outer surface - nickel metal shell, conductor with good corrosion resistance.

Zinc air battery using
The key to the preservation of zinc air battery is the seal. Unless the battery is ready to be used immediately, the battery cathode seal cannot be removed. The simulation test shows that at room temperature, the power will drop to 95% after one year of storage, 90% after two years of storage, and 85% after four years of storage.
After tearing off the seal, the battery is activated and starts to work. When the battery is not connected to a load at room temperature, the battery power will drop by 50% after 3 to 12 weeks, and the power will drop to 0-10% after 20 weeks, depending on the size of the battery. Therefore, zinc air battery are suitable for occasions where the battery will be exhausted within a few weeks.
Once the seal of the zinc air battery is torn off, air will enter the interior to activate the electrochemical reaction. Even if the seal is attached again, the electrochemical reaction will continue until the battery is exhausted.
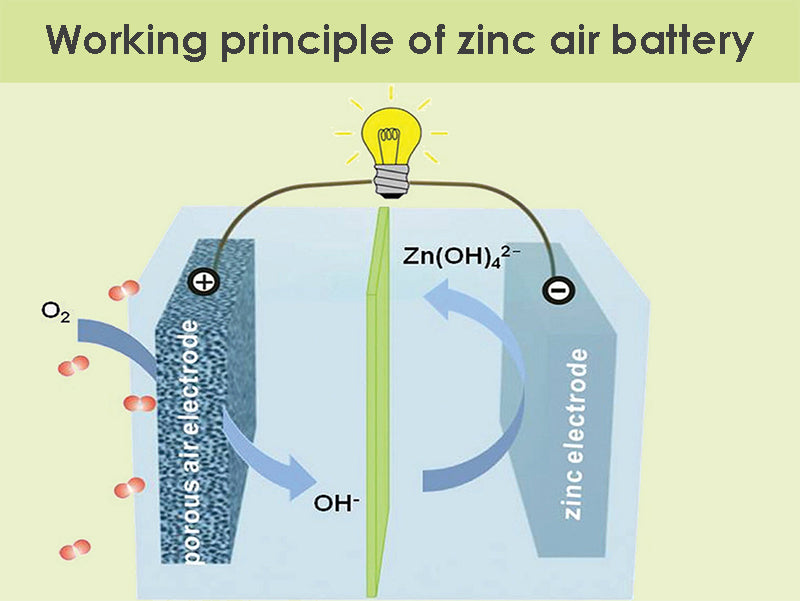
Working principle
The following is the chemical equation for a zinc air battery:
Anode: 2Zn + 4OH– → 2Zn(OH)2 + 4e–
Cathode: O2 + 2H2O + 4e– → 4OH–
Synthesis: 2Zn + O2 → 2ZnO
Usually the voltage generated by this reflection is 1.4 volts, but the discharge current and depth of discharge can cause voltage changes. Air must be able to enter the cathode continuously, and there are small holes in the cathode casing so that oxygen can enter continuously to make the battery produce chemical reactions.
The electrochemical reaction of zinc air battery is as follows:
In neutral solution: 2Zn+4NH4Cl+O2→2Zn(NH3)2Cl2+2H2O
In alkaline solution: 2Zn+2NaOH+O2→2NaHZnO2
The charging capacity of the battery is generally more than 3 times larger than that of the zinc-manganese battery of the same volume. Here is an article of battery capacity for you to gain more knowledge about it.The charge capacity of large zinc air battery is generally 500-2000Ah, and they are mainly used in railway and navigation light devices. Button-shaped zinc air battery have a charge capacity of 200-400mAh and have been widely used in hearing aids.
Different types of zinc-air battery
There are mainly 4 types of zinc air battery.
- Neutral zinc air battery: The structure is similar to that of the zinc-manganese cylindrical battery, and ammonium chloride and zinc chloride are also used as electrolytes, but activated carbon is used instead of manganese dioxide in the carbon bag, and it is placed on or around the cover. There are ventilation holes, which are opened during use;
- Button-type zinc air battery: the structure is basically the same as the zinc-silver button battery, but there is a small hole on the positive shell, which can be opened when in use;
- Zinc-air wet battery: with low power and high charge capacity. It combines sintered or bonded activated carbon electrodes and plate-shaped zinc electrodes to form an electrode group and immerse it in a container filled with sodium hydroxide solution;
- High-power zinc air battery: Generally, the sheet-like bonded activated carbon electrode is installed on the outer wall of the battery, and the zinc powder electrode is installed in the middle of the battery. Potassium hydroxide solution is injected when used. This battery is portable. Low-power zinc-air wet battery and high-power zinc air battery are temporarily activated, and the activated carbon electrode can be used repeatedly. Therefore, after the battery is exhausted, it can be reused as long as the zinc electrode and lye are replaced.
Development status of zinc air battery

Research status of zinc-air primary batteries
Since the development of zinc air battery, its related technologies have been continuously improved, and its performance has also been significantly improved. The main product forms are zinc-air primary batteries and zinc-air secondary batteries, which can meet the needs of various purposes.
Compared with other primary batteries, zinc air battery have the advantages of large capacity, high energy density, low price, and smooth discharge curve. At present, in addition to common button batteries, disposable battery products are increasingly diversified, and the application fields are correspondingly expanded.
- Button type primary battery
Early commercial zinc air battery were limited to button-type primary batteries, which were safe and inexpensive. Used in portable electronic products, mainly hearing aids. At present, the use of this battery is expanding, because the high energy density can gradually meet the general requirements of various uses, such as portable electronic information products such as electronic watches, calculators, and electronic dictionaries.
- Primary batteries of other structures
With the gradual resolution of the leakage prevention problem of cylindrical battery, the production and promotion of practical cylindrical series zinc air battery can be realized. In addition, research on square batteries used in mobile phones has also achieved good results in some domestic research institutes. Israel ElectricFuel was the first to push zinc air battery to the field of mobile phone applications.
Compared with li-ion vs ni-mh battery, this battery has shown significant advantages in terms of price, weight. Scientists have also developed a zinc air battery with a square structure for mobile phones. The specific energy of the battery pack is as high as 220Wh/kg, and the maximum output power can reach 3.6W, and its comprehensive performance fully meets the requirements of mobile phones.
In addition, zinc-air storage batteries have also received attention. This kind of battery is relatively small in size and large in energy storage, and is widely used in military devices, navigation lighting, and field exploration.
Research status of zinc-air secondary batteries
The rapid development of electric vehicles in recent years provides a broad space for the development of zinc air battery. Zinc air battery have the characteristics of high energy, zero pollution, non-combustibility, and recyclability. They are very suitable for use as power sources for urban electric vehicles and have great development potential.
Zinc-air secondary batteries have also become the research and development of various research institutions. focus. Zinc-air secondary batteries mainly include direct rechargeable zinc air battery, mechanically replaceable zinc air battery, and zinc-air fuel cells.
- Connect to a rechargeable zinc air battery
The direct rechargeable zinc air battery directly charges the zinc electrode of the zinc air battery, and there are many problems in the process. For example, the electrochemical activity of zinc in alkaline solution is very high, and the thermodynamic properties are unstable at the same time.
The charging product zincate has a high solubility in strong alkaline solution, so the electrode is prone to deformation, dendrite growth, self-corrosion and passivation, etc. phenomenon, leading to the gradual failure of the electrode.
- Mechanically rechargeable zinc air battery
In view of the difficulty in solving the problems existing in the direct rechargeable zinc air battery, the researchers established a new charging concept of the secondary zinc air battery——mechanical charging method according to the discharge characteristics of the zinc air battery and its own characteristics during the research process.
The so-called mechanical charging means that after the battery is fully discharged, the used zinc electrode in the battery is taken out and replaced with a new zinc electrode, or the entire battery is completely replaced. The whole process takes 3-5 minutes.
Zinc electrode gas batteries can be purchased in supermarkets, battery outlets, auto parts stores, etc., which is very beneficial to the popularization of electric vehicles powered by zinc air battery. The used zinc electrodes or zinc air battery are recycled and reprocessed in a special zinc recycling plant to achieve green environmental protection and pollution-free.
- Zinc-air fuel cell
The concept of zinc-air fuel cell is proposed based on the existing fuel cell. The basic principle is similar to that of mechanical charging, which is to replace the zinc active material. The zinc-air fuel cell is to continuously feed the prepared zinc paste into the battery, and at the same time discharge the reacted mixture out of the battery, and at the same time ensure that the battery has sufficient oxygen supply during operation.
The charging capacity of the battery is generally more than 3 times larger than that of the zinc-manganese battery of the same volume. The charge capacity of large zinc air battery is generally 500-2000Ah, and they are mainly used in railway and navigation light devices. Button-shaped zinc air battery has a charge capacity of 200-400mAh and have been widely used in hearing aids.
Related article: top 10 hydrogen fuel cell companies in China, carbon zinc battery vs alkaline, battery state of charge