
Main content:
In the current commercial production of lithium-ion batteries, the cost of cathode materials accounts for about 40% of the entire battery cost. The price of cathode materials directly determines the price of lithium-ion batteries, especially for lithium-ion power batteries. For example, a small lithium-ion battery for a mobile phone only needs about 5g of cathode material, while a lithium-ion power battery for driving a bus may require up to 500kg of cathode material.
To measure the quality of the cathode material of lithium ion battery, it can be roughly carried out from the following aspects: ① The cathode material should have a higher redox potential, so that the battery has a higher output voltage; Reversible intercalation and deintercalation, so that the battery has high capacity; ③ During the lithium ion intercalation/deintercalation process, the structure of the cathode material should not change or change as little as possible to ensure good cycle performance of the battery: ①i The redox potential of the cathode should change as little as possible during the intercalation/deintercalation process of lithium ions, so that the voltage of the battery will not change significantly, so as to ensure the battery is charged and discharged smoothly; ⑤ The cathode material should have high conductivity , can charge and discharge the battery with high current; ⑥ The cathode does not react chemically with the electrolyte, etc.; ⑦ The lithium ion should have a large diffusion coefficient in the electrode material, which is convenient for the rapid charging and discharging of the battery; ⑧ The price is cheap and no pollution to the environment .
Lithium-ion battery cathode materials are generally lithium oxides, and LiCoO2, LiNiO2, LiMn2O2, LiFePO4 and vanadium oxides are more studied. Conductive polymer cathode materials are also of great interest.
1. Lithium-ion battery whose cathode material is LiCoO2
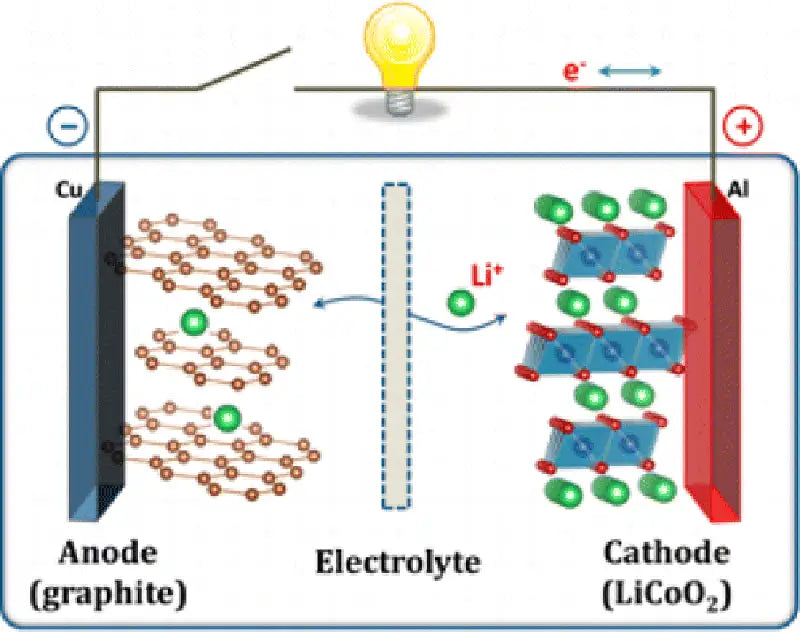
In the current commercial lithium-ion batteries, LiCoO2 with a layered structure is basically selected as the cathode material. Its theoretical capacity is 274mA`h/g, the actual capacity is about 140mA`h/g, and there are reports that the actual capacity has reached 155mA`h/g. The main advantages of the cathode material are: high working voltage, stable charge and discharge voltage, suitable for high current charge and discharge, high specific energy, good cycle performance, high conductivity, simple production process, easy preparation, etc. The main disadvantages are: high price, poor resistance to overcharge, and cycle performance needs to be further improved.
2. Lithium-ion battery with cathode material LiNiO2
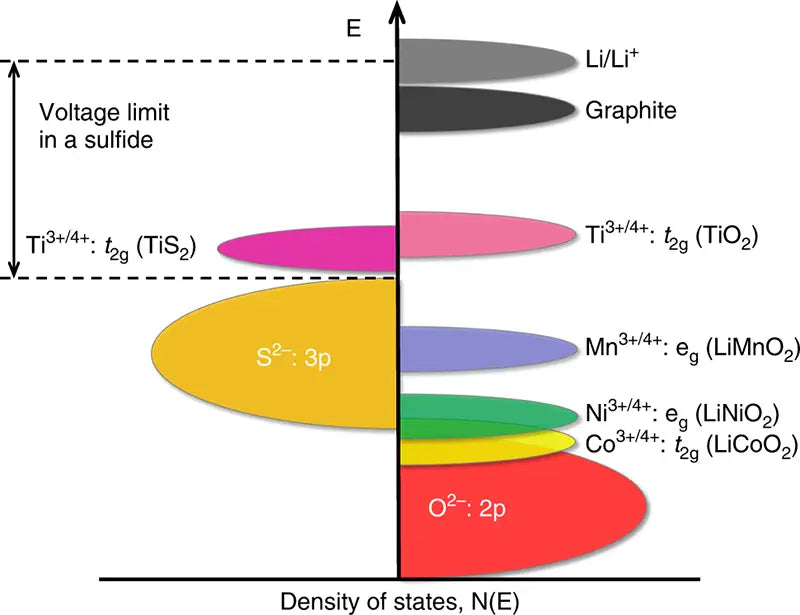
LiNiO2, which is used as a cathode material for lithium-ion batteries, has a layered structure similar to that of LiCO2. Its theoretical capacity is 274mA·h/g, and the actual capacity has reached 190~210mA·h/g. The working voltage range is 2.5~4.2V. The main advantages of this cathode material are: low self-discharge rate, no pollution, good compatibility with various electrolytes, and cheaper price compared to LiCoO2. However, LiNiO2 has fatal shortcomings: the preparation conditions are very harsh, which brings considerable difficulties to the commercial production of LiNiO2; the thermal stability is poor. Compared with LiCoO2 and LiMn2O4 cathode materials under the same conditions, LiNiO2 has the lowest thermal decomposition temperature, daily The amount of heat released is the most, which brings a great safety hazard to the battery; LiNiO2 is prone to structural changes during the charging and discharging process, which deteriorates the cycle performance of the battery. These shortcomings make LiNiO2 still have a long way to go as a cathode material for Li-ion batteries.
3. Lithium-ion battery with cathode material LiMn2O4
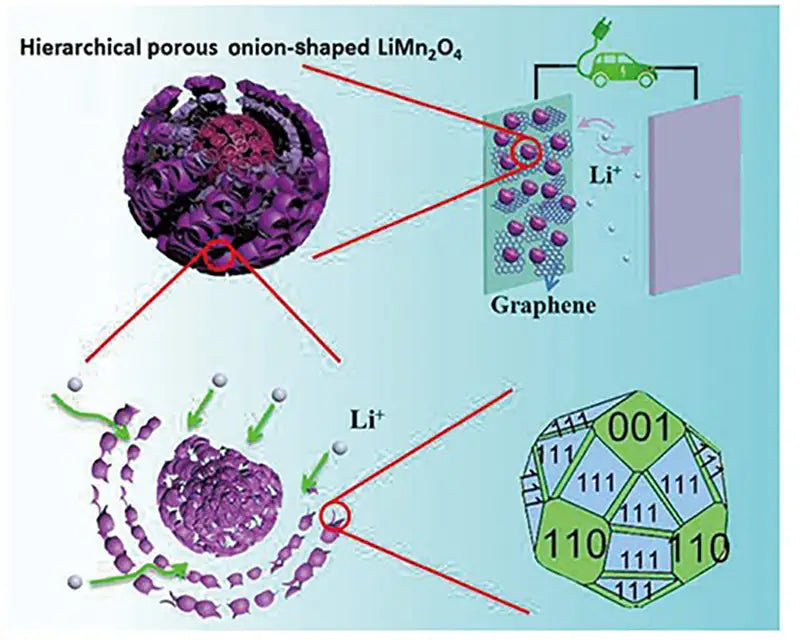
LiMn2O4, which is used as a cathode material for lithium-ion batteries, has a spinel structure. Its theoretical capacity is 148mA·h/g, and the actual capacity is 90~120mA·h/g. The working voltage range is 3~4V. The main advantages of the cathode material are: abundant manganese resources, low price, high safety, and relatively easy preparation. The disadvantage is that the theoretical capacity is not high; the material will dissolve slowly in the electrolyte, that is, the compatibility with the electrolyte is not very good; in the process of deep charge and discharge, the material is prone to lattice distortion, causing the battery capacity to decay rapidly, especially in This is especially true when used at higher temperatures. In order to overcome the above shortcomings, a layered manganese oxide LiMnO2 has been newly developed in recent years. The theoretical capacity of the cathode material is 286mA·h/g, and the actual capacity has reached about 200mA·h/g. The working voltage range is 3~4.5V. Although compared with the spinel-structured LiMn2O4, LiMnO2 has a large improvement in both theoretical capacity and actual capacity, but there is still the problem of structural instability during charging and discharging. During the charging and discharging process, the crystal structure changes repeatedly between the layered structure and the spinel structure, which causes the repeated expansion and contraction of the electrode volume, resulting in the deterioration of the battery cycle performance. Moreover, LiMnO2 also has the problem of dissolution at higher working temperature. The solution to these problems is to doping and surface modification of LiMnO2, and gratifying progress has been made.
4. Lithium-ion battery with cathode material LiFePO4
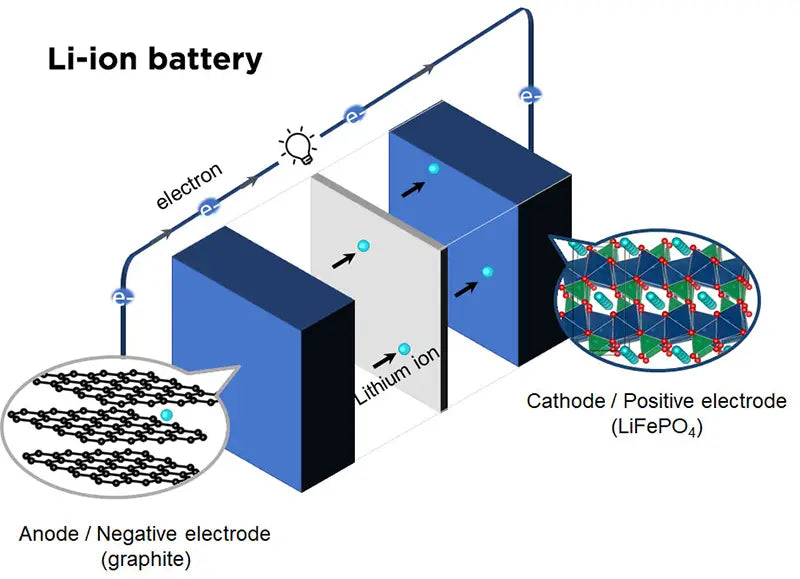
The cathode material has an olivine crystal structure and is one of the popular cathode materials for lithium-ion batteries studied in recent years. Its theoretical capacity is 170mA·h/g, and its actual capacity is as high as 110mA·h/g without doping. By surface modification of LiFePO4, its actual capacity can be as high as 165 mA h/g, which is very close to the theoretical capacity. The operating voltage range is around 3.4V. Compared with the cathode materials introduced above, LiFePO4 has the advantages of high stability, safety, reliability, environmental protection, and low price. The main disadvantages of LiFePO4 are its low theoretical capacity and low room temperature conductivity. Based on the above reasons, LiFePO4 has very good application prospects in large-scale lithium-ion batteries. However, to show strong market competitiveness in the entire lithium-ion battery field, LiFePO4 faces the following unfavorable factors: ①Low-cost competition from LiMn2O4, LiMnO2, LiNiMO2 cathode materials; ②In different application fields, people may prefer to choose more suitable ③LiFePO4’s battery capacity is not high; ④In high-tech fields, people are more concerned about performance rather than cost, such as in mobile phones and notebook computers; ⑤LiFePO4 urgently needs to improve its electrical conductivity during deep discharge at 1C speed , in order to improve its specific capacity; ⑤ In terms of safety, LiCoO2 represents the current safety standard in the industry, and the safety of LiNiO2 has also been greatly improved, while LiFePO4 only shows higher safety performance, especially in Electric vehicles and other aspects can ensure their full competitive advantage in safety.
Although there are many kinds of cathode materials that can theoretically be used as lithium-ion batteries, LiCoO2 is still the most widely used cathode material in commercial lithium-ion batteries. Although the layered LiNiO2 has a higher specific capacity than LiCoO2, due to its structural changes and safety problems caused by its thermal decomposition reaction, there is still a considerable distance to directly apply LiNiO2 as a cathode material. Partially replacing Ni with Co to obtain LiNi1-xCoxO2 with higher safety as a cathode material may be an important development direction in the future. Spinel-structured LiMn2O4 and layered-structured LiMnO2 are considered as one of the most competitive cathode candidates due to their abundant raw material resources, obvious price advantage and high safety. However, the problem of structural instability during the charging and discharging process is an important research topic in the future. The current actual discharge capacity of LiFePO4 with morinite structure has reached about 95% of the theoretical capacity, and it has the advantages of low price, high safety, stable structure, and no environmental pollution. It is considered to be very promising in large lithium-ion batteries. However, the theoretical capacity limitation of LiFePO4 makes it unlikely to be used in high-capacity small lithium-ion batteries.